Os testes no local de atendimento (POCT) têm um impacto profundo nos cuidados de saúde, oferecendo soluções de diagnóstico rápidas e convenientes. É amplamente utilizado em ambientes clínicos, departamentos de emergência e áreas remotas ou carentes para testes no local. O POCT ajuda na detecção precoce de doenças, no monitoramento de condições crônicas e na tomada de decisões oportunas de tratamento. Também reduz a necessidade de testes laboratoriais, resultando em resultados mais rápidos e melhores resultados para os pacientes. Sua versatilidade torna o POCT inestimável no gerenciamento de condições como diabetes, doenças infecciosas, problemas cardíacos e muito mais.
Acesse o relatório completo @ https://www.databridgemarketresearch.com/reports/apac-point-care-testing-poct-market
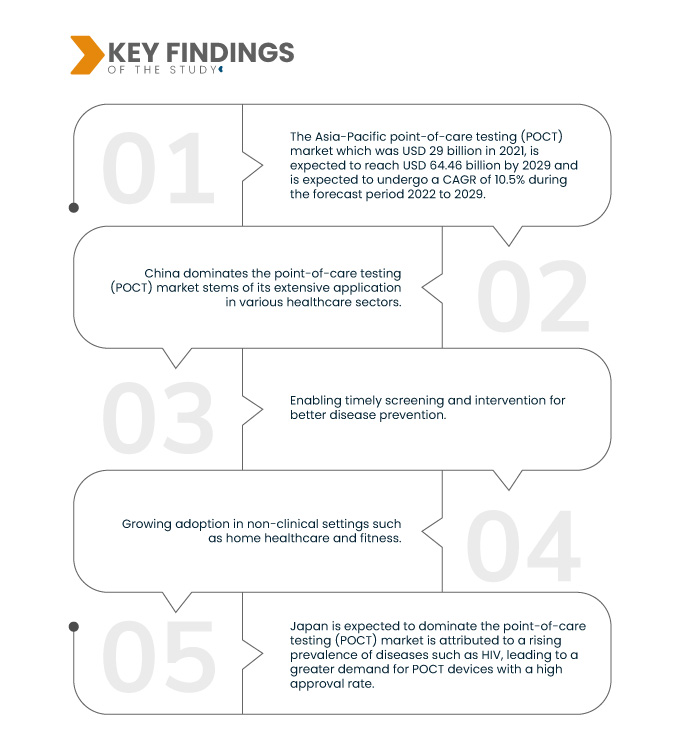
A Data Bridge Market Research analisa que o Mercado Ásia-Pacífico de testes no ponto de atendimento (POCT) que foi de US$ 29 bilhões em 2021, deverá atingir US$ 64,46 bilhões até 2029, e deverá passar por um CAGR de 10,5% durante o período de previsão de 2022 a 2029. Iniciativas governamentais e aprovações para dispositivos de diagnóstico rápido reforçam o crescimento do mercado, promovendo um ambiente regulatório propício e incentivo à inovação. Este apoio acelera o desenvolvimento e a adoção de soluções de testes no local de atendimento, melhorando a acessibilidade e os resultados dos cuidados de saúde.
Principais conclusões do estudo
Espera-se que áreas remotas e mal atendidas impulsionem a taxa de crescimento do mercado
Os testes no local de atendimento (POCT) são cruciais em regiões com instalações de saúde limitadas, pois fornecem capacidades de diagnóstico imediato. Em áreas sem acesso a hospitais ou laboratórios bem equipados, os dispositivos POCT oferecem testes no local, permitindo uma identificação, tratamento e intervenção mais rápidos da doença. Isto reduz a carga sobre as instalações de saúde centralizadas e os profissionais de saúde, melhorando o acesso geral e a qualidade dos cuidados de saúde em regiões remotas ou mal servidas, melhorando, em última análise, os resultados de saúde e colmatando as disparidades nos cuidados de saúde.
Escopo do relatório e segmentação de mercado
|
Métrica de relatório
|
Detalhes
|
|
Período de previsão
|
2022 a 2029
|
|
Ano base
|
2021
|
|
Anos históricos
|
2020 (personalizável para 2014-2019)
|
|
Unidades Quantitativas
|
Receita em bilhões de dólares, volumes em unidades, preços em dólares
|
|
Segmentos cobertos
|
Produto (produtos de monitoramento de glicose, produtos para testes cardiometabólicos, produtos para testes de doenças infecciosas, produtos para testes de gravidez e fertilidade, produtos para testes de marcadores de tumor/câncer, produtos para testes de urinálise, produtos para testes de colesterol, produtos para testes de hematologia, produtos para testes de drogas de abuso, ocultação fecal Produtos de teste, teste rápido de coagulação e outros produtos POC), aplicação (transfusão de sangue, monitoramento cardíaco, coagulação, glicemia, Hematologia, monitoramento não invasivo de SPO2, monitoramento não invasivo de PCO2, análise de sangue total, monitoramento de sinais vitais e outros), plataforma (ensaios de fluxo lateral/testes de imunocromatografia, varetas, microfluídica, Diagnóstico Molecular e imunoensaios), modo de prescrição (testes baseados em prescrição e testes OTC), tipo de teste (imunoensaios, ensaios baseados em células, testes de amplificação de ácido nucleico, ensaios de química clínica e hematologia), usuário final (hospitais, clínicas, laboratórios, atendimento domiciliar , Centros de Cirurgia Ambulatorial e Outros), Canal de Distribuição (Concurso Direto e Farmácias de Retalho)
|
|
Países abrangidos
|
China, Japão, Índia, Coreia do Sul, Singapura, Malásia, Austrália, Tailândia, Indonésia, Filipinas, Resto da Ásia-Pacífico (APAC) na Ásia-Pacífico (APAC)
|
|
Participantes do mercado cobertos
|
Abbott (EUA), F. Hoffmann-La Roche Ltd (Suíça), BD (EUA), Siemens Healthcare Private Limited (Alemanha), Bio-Rad Laboratories, Inc. (EUA), Danaher (EUA), Sekisui Diagnostics (EUA) , Trinity Biotech (Irlanda), bioMérieux (França), EKF Diagnostics (Alemanha), AccuBioTech Co., Ltd. (China), Instrumentation Laboratory (EUA), Beckman Coulter, Inc. Biomedical (EUA), Chembio Diagnostics, Inc. (EUA), Quidel Corporation (EUA) e Sienco, Inc (EUA)
|
|
Pontos de dados abordados no relatório
|
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e grandes players, os relatórios de mercado com curadoria da Data Bridge Market Research também incluem análise especializada aprofundada, epidemiologia de pacientes, análise de pipeline, análise de preços, e quadro regulamentar.
|
Análise do segmento:
O mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado com base no produto, tipo de teste, prescrição, plataforma, aplicação, usuário final e canal de distribuição.
- Com base no produto, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em produtos de monitoramento de glicose, produtos de testes cardiometabólicos, produtos de testes de doenças infecciosas, produtos de testes de gravidez e fertilidade, produtos de testes de marcadores tumorais/câncer, urinálise produtos para testes, produtos para testes de colesterol, produtos para testes de hematologia, produtos para testes de drogas de abuso, produtos para testes de ocultismo fecal, testes rápidos de coagulação e outros produtos POC
- Com base no tipo de teste, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em imunoensaios, ensaios baseados em células, testes de amplificação de ácidos nucleicos, ensaios de química clínica e hematologia.
- Com base na prescrição, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em testes baseados em prescrição e testes OTC.
- Com base na plataforma, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em (ensaios de fluxo lateral/testes de imunocromatografia, varetas, microfluídica, diagnóstico molecular e imunoensaios.
- Com base na aplicação, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em transfusão de sangue, monitoramento cardíaco, coagulação, glicemia, hematologia, monitoramento não invasivo de spo2, monitoramento não invasivo de pco2, sangue total análise, monitoramento de sinais vitais e outros
- Com base no usuário final, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em hospitais, clínicas, laboratórios, atendimento domiciliar, centros de cirurgia ambulatorial, entre outros.
- Com base no canal de distribuição, o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico é segmentado em concurso direto e farmácias de varejo.
Jogadores principais
A Data Bridge Market Research reconhece as seguintes empresas como participantes do mercado de testes no ponto de atendimento (POCT) da Ásia-Pacífico no mercado de testes no ponto de atendimento (POCT) da Ásia-Pacífico são Abbott (EUA), F. Hoffmann-La Roche Ltd (Suíça), BD (EUA), Siemens Healthcare Private Limited (Alemanha), Bio-Rad Laboratories, Inc. (EUA), Danaher (EUA), Sekisui Diagnostics (EUA).
Desenvolvimentos de mercado
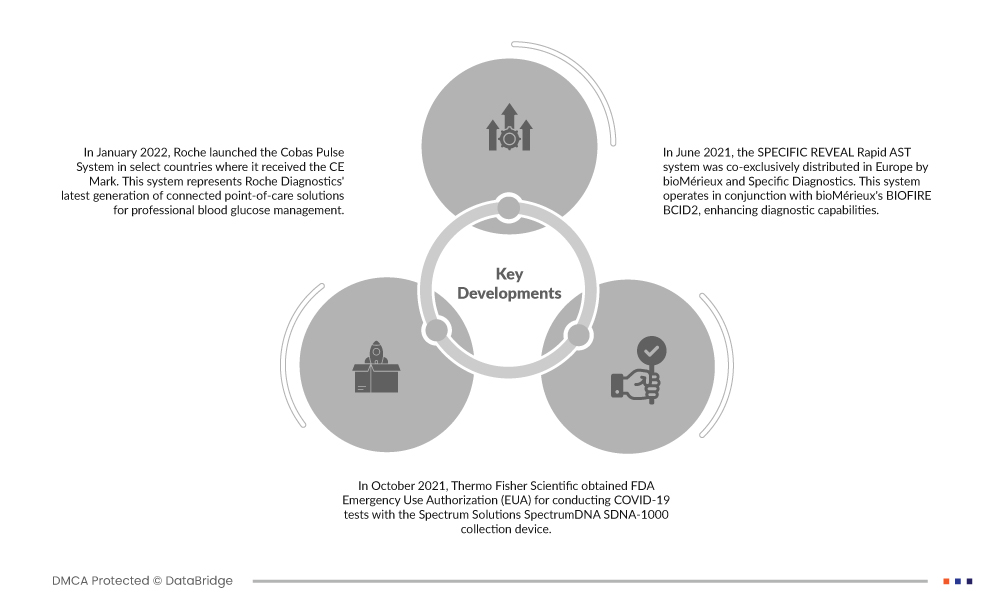
- Em janeiro de 2022, a Roche lançou o Sistema Cobas Pulse em países selecionados onde recebeu a Marca CE. Este sistema representa a última geração de soluções conectadas no local de atendimento da Roche Diagnostics para gerenciamento profissional de glicose no sangue. A Marca CE significa conformidade com os padrões da União Europeia para dispositivos médicos, indicando a adesão do produto aos padrões de qualidade e segurança. O Cobas Pulse System visa fornecer soluções avançadas de monitorização da glicemia para profissionais de saúde e pacientes.
- Em outubro de 2021, a Thermo Fisher Scientific obteve Autorização de Uso de Emergência (EUA) da FDA para conduzir testes de COVID-19 com o dispositivo de coleta Spectrum Solutions SpectrumDNA SDNA-1000. Este sistema de teste automatizado de alto rendimento, integrado à Solução Amplitude, introduziu um novo método de coleta de amostras de saliva para testes de COVID-19. A aprovação dos EUA marcou um passo importante na melhoria das capacidades de teste da COVID-19, tornando o processo mais eficiente e acessível ao público.
- Em junho de 2021, o sistema SPECIFIC REVEAL Rapid AST foi distribuído co-exclusivamente na Europa pela bioMérieux e Specific Diagnostics. Este sistema funciona em conjunto com o BIOFIRE BCID2 da bioMérieux, melhorando as capacidades de diagnóstico. Ele permite testes de suscetibilidade antimicrobiana (AST) rápidos e precisos, auxiliando os médicos a determinar o tratamento mais eficaz para pacientes com infecções da corrente sanguínea. A colaboração entre as duas empresas procurou fornecer aos profissionais de saúde na Europa ferramentas avançadas para a gestão de infecções.
Análise Regional
Geograficamente, os países cobertos no relatório de mercado de testes no local de atendimento (POCT) da Ásia-Pacífico são China, Japão, Índia, Coreia do Sul, Cingapura, Malásia, Austrália, Tailândia, Indonésia, Filipinas, Resto da Ásia-Pacífico (APAC ) na Ásia-Pacífico (APAC).
De acordo com a análise da pesquisa de mercado da Data Bridge:
A China é a região dominante no mercado de testes no local de atendimento (POCT) da Ásia-Pacífico durante o período de previsão 2022-2029
A China domina o mercado de testes no local de atendimento (POCT) decorre de sua ampla aplicação em vários setores de saúde. Os produtos POCT são utilizados em operações hospitalares, departamentos de emergência, terapia intensiva, gerenciamento de doenças crônicas, resposta a crises de saúde pública, desenvolvimento de instalações de saúde em nível de condado e apoio à saúde rural. Esta ampla gama de aplicações posiciona a China como um ator-chave no avanço das soluções POCT e no atendimento de diversas necessidades de saúde.
Espera-se que o Japão domine o mercado de testes no local de atendimento (POCT) da Ásia-Pacífico em o período de previsão 2022-2029
Espera-se que o Japão domine o mercado de testes no local de atendimento (POCT), o que é atribuído a uma prevalência crescente de doenças como o HIV, levando a uma maior demanda por dispositivos POCT com uma alta taxa de aprovação. Em contraste, a proeminência da Índia deve-se ao seu fardo substancial de doenças, impulsionando um mercado robusto para soluções POCT. Estes países destacam-se como contribuintes significativos para o crescimento global do POCT, atendendo a necessidades específicas de cuidados de saúde.
Para obter informações mais detalhadas sobre o relatório de mercado de testes no local de atendimento (POCT) da Ásia-Pacífico, clique aqui –