Тестирование в месте оказания медицинской помощи (POCT) производит революцию в предоставлении медицинских услуг, обеспечивая быструю диагностику на месте нахождения пациента. Его применение охватывает различные медицинские учреждения, включая клиники, больницы и отдаленные районы. Устройства POCT предлагают удобные интерфейсы, выдавая быстрые и точные результаты для таких состояний, как диабет, сердечные маркеры и инфекционные заболевания . Эти функции улучшают уход за пациентами, облегчая принятие немедленных решений о лечении, сокращая время выполнения и расширяя доступ к здравоохранению в недостаточно обслуживаемых регионах, в конечном итоге оптимизируя медицинские результаты.
Доступ к полному отчету по адресу https://www.databridgemarketresearch.com/reports/europe-poct-market
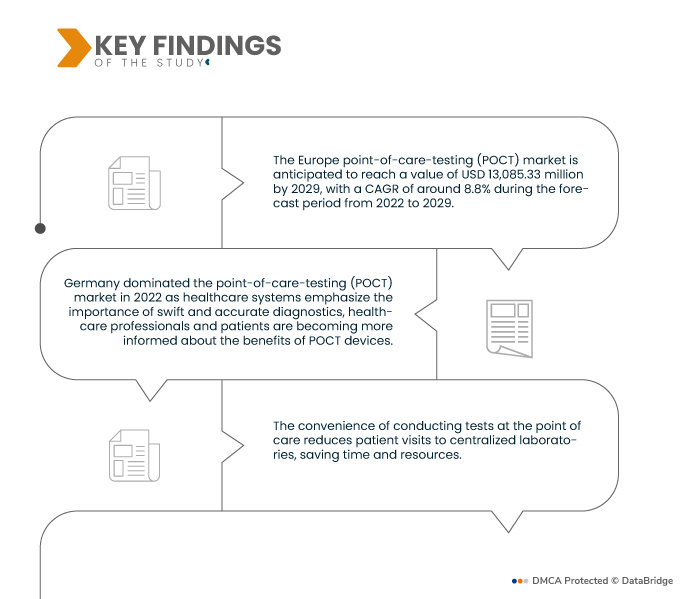
Data Bridge Market Research анализирует, что рынок тестирования в точках оказания медицинской помощи (POCT) в Европе оценивается в 6 664,28 млн долларов США в 2021 году и, как ожидается, достигнет 13 085,33 млн долларов США к 2029 году, регистрируя среднегодовой темп роста 8,8% в прогнозируемый период с 2022 по 2029 год. Тестирование в точках оказания медицинской помощи (POCT) обеспечивает быстрые диагностические результаты непосредственно на месте нахождения пациента, позволяя медицинским работникам принимать немедленные решения о лечении. Этот быстрый доступ к критически важной информации улучшает уход за пациентами, сводя к минимуму задержки в диагностике и вмешательстве, что приводит к более своевременному и эффективному медицинскому управлению.
Основные выводы исследования
Ожидается, что ранняя диагностика и вмешательство будут способствовать темпам роста рынка.
Тестирование в месте оказания помощи (POCT) играет ключевую роль в раннем выявлении заболеваний, позволяя быстро начинать лечение и в конечном итоге приводя к лучшим результатам для пациентов. Предоставляя быструю и оперативную диагностическую информацию, специалисты здравоохранения могут оперативно вмешиваться с соответствующими методами лечения, предотвращая прогрессирование заболевания и осложнения. Этот проактивный подход значительно повышает эффективность медицинских вмешательств, улучшает прогноз для пациента и способствует общей эффективности системы здравоохранения.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2022-2029
|
Базовый год
|
2021
|
Исторические годы
|
2020 (Можно настроить на 2014-2019)
|
Количественные единицы
|
Доход в млн. долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Продукция (инструменты, расходные материалы и реагенты и другие), Применение (глюкоза в крови, мониторинг сердца, коагуляция, анализ цельной крови, мониторинг основных показателей жизнедеятельности, тестирование на инфекционные заболевания, анализ мочи, тестирование на холестерин и другие), Конечный пользователь (лаборатории, больницы, клиники, амбулаторные центры, уход на дому, дома престарелых, аптеки и другие), Способ тестирования (тестирование по рецепту и безрецептурное тестирование), Канал распространения (прямые продажи, сторонние дистрибьюторы и онлайн-продажи)
|
Страны, охваченные
|
Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, Остальная Европа в Европе
|
Охваченные участники рынка
|
Siemens Healthcare GmbH (Германия), F. Hoffmann-La Roche Ltd. (Швейцария), Quidel Corporation (США), Trividia Health, Inc. (США), Nova Biomedical (США), PTS Diagnostics (США), Trinity Biotech (Ирландия), LumiraDx (Великобритания), Sekisui Diagnostics (США), Chembio Diagnostics, Inc. (США), BD (США), Thermo Fisher Scientific Inc. (США), Abbott (США), Danaher (США), binx health, inc (США), QuantuMDx Group Ltd. (Великобритания), Werfen (Испания), Abaxis (США), EKF Diagnostics (Великобритания), Sysmex Europe SE (Германия)
|
Данные, отраженные в отчете
|
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя глубокий экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Европейский рынок тестирования в местах оказания медицинской помощи (POCT) сегментирован по продукту, области применения, конечному пользователю, способу тестирования и каналу сбыта.
- По продуктовому признаку рынок сегментируется на инструменты, расходные материалы и реагенты и т. д.
- По области применения рынок сегментирован на анализы на уровень глюкозы в крови, кардиомониторинг, коагуляцию, анализ цельной крови, мониторинг основных показателей жизнедеятельности, тестирование на инфекционные заболевания, анализ мочи, тестирование на холестерин и другие.
- По типу конечного пользователя рынок сегментируется на лаборатории, больницы, клиники, амбулаторные центры, учреждения по уходу на дому, дома престарелых, аптеки и другие.
- По способу тестирования рынок сегментируется на тестирование по рецепту и безрецептурное тестирование.
- По каналу сбыта рынок сегментируется на прямые продажи, сторонних дистрибьюторов и онлайн-продажи.
Основные игроки
Data Bridge Market Research признает следующие компании основными игроками на европейском рынке тестирования в пунктах оказания медицинской помощи (POCT): Siemens Healthcare GmbH (Германия), F. Hoffmann-La Roche Ltd. (Швейцария), Quidel Corporation (США), Trividia Health, Inc. (США), Nova Biomedical (США), PTS Diagnostics (США), Trinity Biotech (Ирландия), LumiraDx (Великобритания),
Развитие рынка
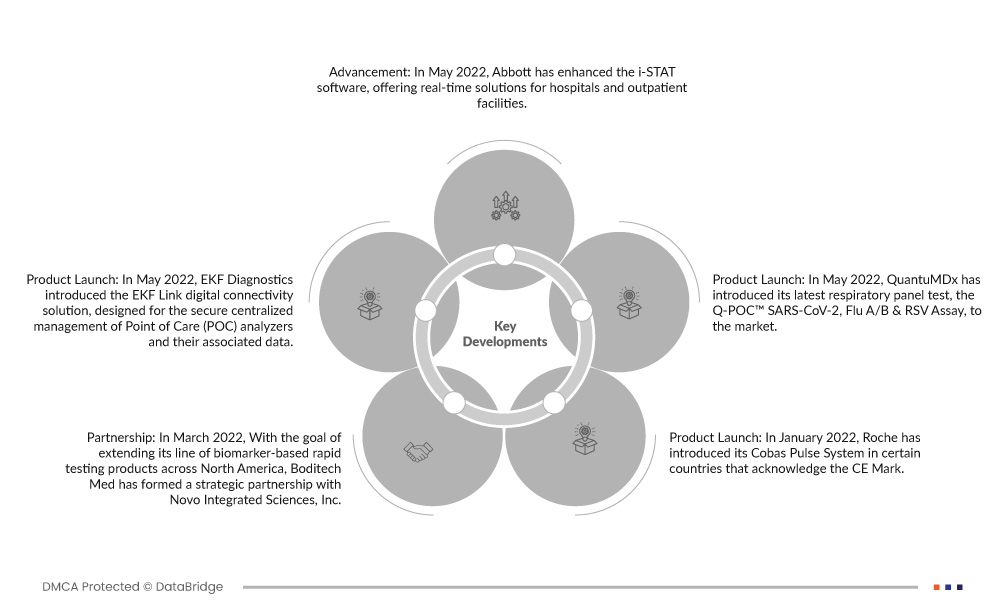
- В мае 2022 года компания Abbott усовершенствовала программное обеспечение i-STAT, предлагая решения в режиме реального времени для больниц и амбулаторных учреждений. Это обновленное программное обеспечение отличается высокой эффективностью, экономической эффективностью и поддерживает качественное принятие решений по уходу за пациентами, гарантируя эффективные и точные решения в области здравоохранения.
- В мае 2022 года компания EKF Diagnostics представила решение для цифрового подключения EKF Link, предназначенное для безопасного централизованного управления анализаторами Point of Care (POC) и связанными с ними данными. Эта платформа оптимизирует процесс управления, обеспечивая безопасность данных и повышая эффективность операций по тестированию POC.
- В мае 2022 года компания QuantuMDx представила на рынке новейший респираторный тест-панель — Q-POC™ SARS-CoV-2, Flu A/B & RSV Assay.
- В марте 2022 года с целью расширения линейки продуктов для экспресс-тестирования на основе биомаркеров в Северной Америке компания Boditech Med заключила стратегическое партнерство с Novo Integrated Sciences, Inc.
- В январе 2022 года компания Roche представила свою систему Cobas Pulse в некоторых странах, признающих маркировку CE. Это передовое предложение от Roche Diagnostics представляет собой их новейшее решение для подключенных точек оказания помощи, разработанное для профессионального управления уровнем глюкозы в крови.
Региональный анализ
Географически в отчете о рынке тестирования в местах оказания медицинской помощи (POCT) в Европе представлены следующие страны: Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция и остальные страны Европы.
Согласно анализу Data Bridge Market Research:
Германия будет доминирующим регионом на европейском рынке тестирования в местах оказания медицинской помощи (POCT) в прогнозируемый период 2022–2029 гг.
В 2022 году Германия доминировала на рынке тестирования в месте оказания медицинской помощи (POCT) благодаря растущей осведомленности о преимуществах тестирования на месте, предлагая быстрые результаты, которые улучшают уход за пациентами, ускоряют выбор лечения и повышают эффективность управления заболеваниями. Это возросшее знакомство с возможностями POCT способствует его принятию в качестве основополагающего элемента современной практики здравоохранения по всей Европе.
Более подробную информацию об отчете о рынке тестирования в местах оказания медицинской помощи (POCT) можно получить здесь – https://www.databridgemarketresearch.com/reports/europe-poct-market