外傷固定装置は、骨折した骨を安定させ、治癒を促進するために利用されます。これらは、骨折、脱臼、複雑な損傷の治療を含むさまざまな整形外科処置に応用されています。これらのデバイスは、安定性を高めるためのロック機構、正確にフィットするための解剖学的設計、組織の統合を促進するための生体適合性材料などの機能を備えています。外科医は、外科的介入中にプレート、ネジ、ロッド、髄内釘などの外傷固定インプラントを使用して内部サポートを提供し、骨折した骨の位置合わせと治癒を促進し、患者の転帰の改善とより早い回復につながります。
完全なレポートにアクセス: https://databridgemarketresearch.com/reports/us-trauma-fixation-market
データブリッジマーケットリサーチの分析によると、 米国の外傷修復市場 は、2022 年から 2029 年の予測期間中に 6.70% の CAGR を記録しています。転倒事故、スポーツ関連の傷害、外傷性出来事の発生率の増加により、外傷固定装置と処置の必要性が高まっています。これらの損傷には即時かつ効果的な治療が必要であるため、患者にタイムリーで成功した結果を提供するために、外傷固定ソリューションの需要が高まっています。
研究の主な結果
テクノロジーの進歩が市場の成長率を促進すると予想されます
安定性と骨折固定を向上させるロッキングプレート、骨治癒を促進する髄内釘、組織損傷を軽減する低侵襲外科技術などの外傷固定装置の革新は、患者ケアに革命をもたらしました。これらの進歩により、治療結果の向上、回復時間の短縮、術後の合併症の減少がもたらされ、その結果、外傷固定処置および装置の採用が増加し、市場の範囲と可能性が拡大しました。
レポートの範囲と市場セグメンテーション
|
レポートメトリック
|
詳細
|
|
予測期間
|
2022年から2029年
|
|
基準年
|
2021
|
|
歴史的な年
|
2020 (2014~2019年にカスタマイズ可能)
|
|
対象セグメント
|
タイプ (内部固定具、外部固定具、その他の外傷製品)、材質 (ステンレス鋼、チタン、その他)、手術部位 (下肢および上肢)、最終用途 (病院、外傷センター、外来手術センターなど) 、流通チャネル (直接入札および小売)
|
|
対象となる市場関係者
|
Johnson & Johnson Services, Inc. (米国)、Stryker (米国)、B. Braun Melsungen AG (ドイツ)、Medartis AG (スイス)、Orthofix Medical Inc. (米国)、Medtronic (米国)、Orthofix Holdings Inc. (米国) )、Integra LifeSciences (米国)、Cardinal Health (米国)、Accumed LLC (米国)、Corin Underwriting Limited (英国)、KLS Martin Group (ドイツ)、Arthosurface (米国)、ReWalk Robotics (米国)、Advance Orthopedic Solutions (米国) )、Bioretec Ltd.(フィンランド)、Poriferous LLC(米国)、Acumed LLC(米国)、Conmed Corporation(米国)、Smith & Nephew(英国)、Wright Medical Group NV(米国)、Zimmer Biomet Holdings Inc.(米国) )
|
|
レポートで取り上げるデータポイント
|
市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、Data Bridge Market Researchが厳選した市場レポートには、専門家の詳細分析、患者疫学、パイプライン分析、価格分析、および規制の枠組み
|
セグメント分析:
米国の外傷固定市場は、種類、手術部位、材料、流通チャネル、最終用途に基づいて分割されています。
- 米国の外傷固定市場は、タイプに基づいて、内部固定器、外部固定器、およびその他の外傷製品に分類されます。
- 手術部位に基づいて、米国の外傷固定市場は下肢と上肢に分類されます。
- 材質に基づいて、米国の外傷固定市場はステンレス鋼、 チタン、 その他。
- 流通チャネルに基づいて、米国の外傷固定市場は次のように分類されます。 ステンレス鋼、 チタンなど。
- 最終用途に基づいて、市場は病院、外傷センター、 外来手術センター、 その他。
主要プレーヤー
Data Bridge Market Research は、米国の外傷固定市場における主要な米国の外傷固定市場プレーヤーとして以下の企業を認識しています: Johnson & Johnson Services, Inc. (米国)、Stryker (米国)、B. Braun Melsungen AG (ドイツ)、Medartis AG (スイス)、Orthofix Medical Inc.(米国)、Medtronic(米国)、Orthofix Holdings Inc.(米国)、Integra LifeSciences(米国)、Cardinal Health(米国)、Accumed LLC(米国)、Corin Underwriting Limited(英国)、KLSマーティングループ(ドイツ)。
市場の発展
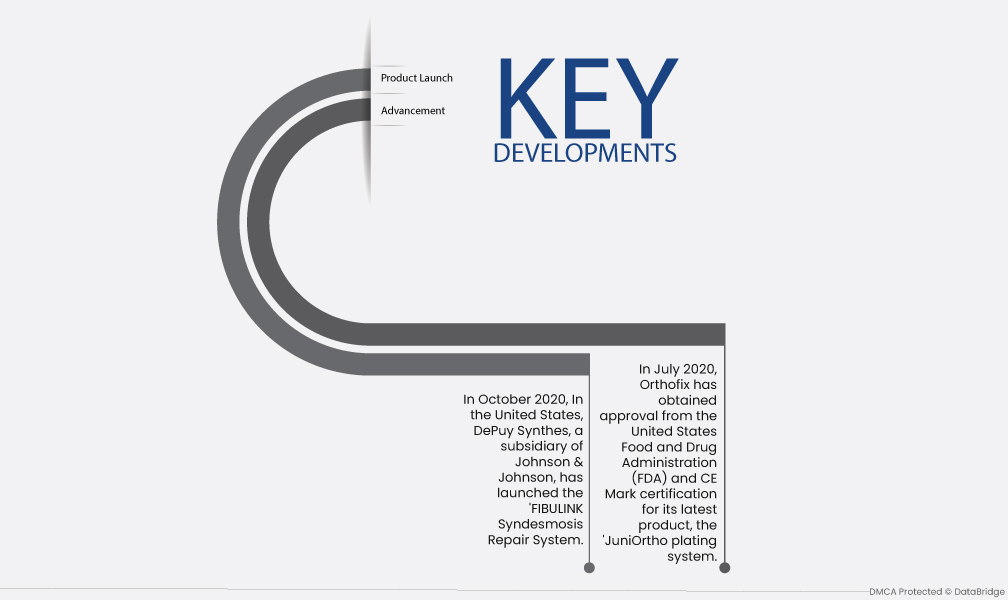
- 2020年10月、米国ではジョンソン・エンド・ジョンソンの子会社であるデピューシンセスが「FIBULINK 靱帯結合組織修復システム」を発売しました。この革新的なデバイスは靱帯結合組織への外傷を効果的に治療することを目的としており、靱帯結合組織損傷の修復を必要とする患者に特化したソリューションを提供します。
- 2020年7月、オルソフィックスは最新製品「JuniOrthoプレーティングシステム」について米国食品医薬品局(FDA)の承認とCEマーク認証を取得した。小児患者向けに調整されたこの革新的なシステムは、下肢の高度な変形と外傷の再建に対処するように設計されており、整形外科的解決を必要とする若い患者に専門的な治療オプションを提供します。
米国外傷固定市場に関する詳細情報 レポートはこちらをクリックしてください –