North America Topical Drug Delivery Market
Taille du marché en milliards USD
TCAC :
% 
 USD
54.23 Billion
USD
91.80 Billion
2024
2032
USD
54.23 Billion
USD
91.80 Billion
2024
2032
| 2025 –2032 | |
| USD 54.23 Billion | |
| USD 91.80 Billion | |
|
|
|
|
Segmentation du marché nord-américain de l'administration topique de médicaments, par type de produit (formulations et dispositifs d'administration de médicaments), techniques d'administration (ionotophorèse, sonophorèse, ablation au laser, ablation par radiofréquence, magnétophorèse, électroporation et autres), type (agents nettoyants, agents protecteurs, agents hydratants, agents desséchants, agents anti-démangeaisons, agents anti-inflammatoires, agents anti-infectieux, kératolytiques et autres), génération (systèmes d'administration transdermique de première génération, systèmes d'administration transdermique de deuxième génération et systèmes d'administration transdermique de troisième génération), mode d'achat (sur ordonnance et en vente libre), indication (troubles dermatologiques, troubles ophtalmiques, gestion de la douleur, troubles neurochirurgicaux, hormonothérapie, sevrage tabagique et autres), voie d'administration (cutanée, ophtalmique, rectale, vaginale, nasale et autres), utilisateur final (hôpitaux, centres spécialisés, soins à domicile et Autres), Canal de distribution (pharmacie hospitalière, pharmacie de détail, pharmacie en ligne et autres) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché nord-américain de l'administration topique de médicaments
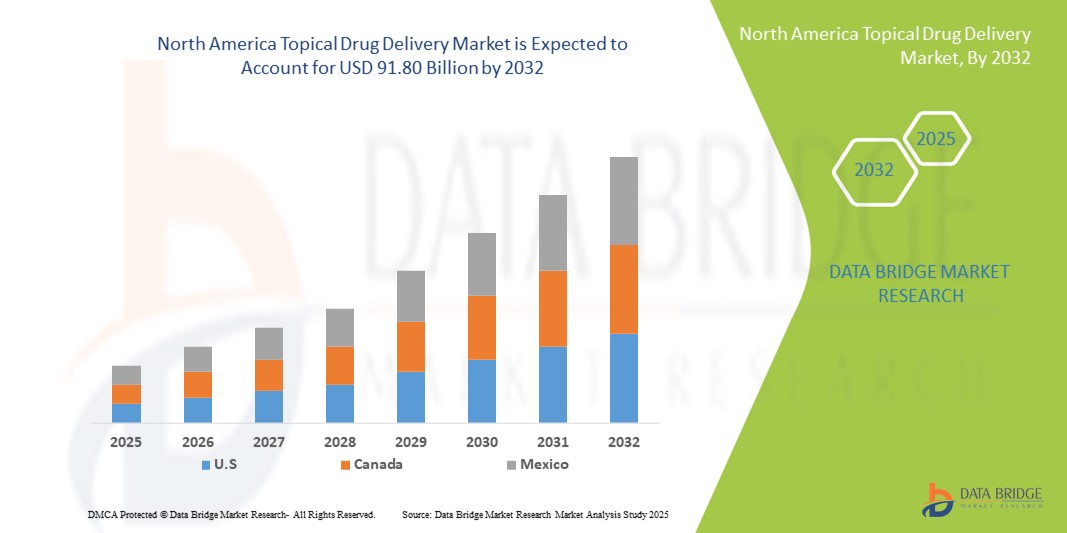
- La taille du marché nord-américain de l'administration topique de médicaments était évaluée à 54,23 milliards USD en 2024 et devrait atteindre 91,80 milliards USD d'ici 2032 , à un TCAC de 6,8 % au cours de la période de prévision.
- Cette croissance est principalement due à la prévalence croissante des troubles cutanés, à la demande croissante d’options de traitement non invasives et aux progrès des technologies d’administration de médicaments.
- En outre, la préférence croissante pour l’auto-administration, en particulier pour les affections cutanées chroniques et la gestion de la douleur, contribue à l’expansion du marché.
Analyse du marché nord-américain de l'administration topique de médicaments
- Les systèmes d'administration topique de médicaments, permettant l'administration localisée d'agents thérapeutiques à travers la peau, sont de plus en plus essentiels en dermatologie et dans la gestion de la douleur en raison de leur nature non invasive, de leur action ciblée et de leurs effets secondaires systémiques réduits.
- La demande croissante d’administration de médicaments topiques est principalement alimentée par la prévalence croissante des troubles cutanés, des douleurs chroniques et de la préférence des patients pour l’auto-administration par rapport aux thérapies orales ou injectables.
- Les États-Unis ont dominé le marché nord-américain de l'administration topique de médicaments avec la plus grande part de revenus de 85,3 % en 2024, caractérisé par une infrastructure de recherche pharmaceutique avancée, des dépenses de santé élevées et une adoption généralisée de technologies innovantes d'administration de médicaments, avec une croissance substantielle des patchs et gels transdermiques tirée par les progrès des systèmes d'administration transdermique de deuxième génération.
- Le Canada devrait être le pays connaissant la croissance la plus rapide sur le marché nord-américain de l’administration de médicaments topiques au cours de la période de prévision en raison de la sensibilisation croissante aux soins de santé, de la prévalence croissante des affections dermatologiques et de l’adoption croissante d’options thérapeutiques non invasives.
- Le segment de l'ionophorèse a dominé le marché nord-américain de l'administration topique de médicaments avec une part de marché de 28,9 % en 2024, grâce à sa capacité à améliorer la pénétration des médicaments de manière non invasive et à améliorer les résultats thérapeutiques.
Portée du rapport et segmentation du marché nord-américain de l'administration topique de médicaments
|
Attributs |
Aperçu du marché nord-américain de l'administration topique de médicaments |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché nord-américain de l'administration topique de médicaments
Adoption croissante de techniques avancées d'accouchement non invasives
- Une tendance importante et croissante sur le marché nord-américain de l’administration topique de médicaments est l’utilisation croissante de techniques non invasives avancées telles que l’ionophorèse, la sonophorèse et les systèmes assistés par micro-aiguilles, qui améliorent la pénétration des médicaments et l’observance du traitement par les patients.
- Par exemple, les patchs d’iontophorèse sont utilisés pour administrer efficacement des agents anti-inflammatoires pour la gestion localisée de la douleur, permettant un dosage précis et réduisant les effets secondaires systémiques.
- L'intégration de la nanotechnologie et des formulations à libération prolongée dans les produits topiques permet une libération contrôlée du médicament, améliore l'efficacité thérapeutique et offre une action prolongée, augmentant ainsi le confort du patient.
- Ces avancées facilitent la thérapie personnalisée pour les affections dermatologiques chroniques et liées à la douleur, faisant de l'administration topique une alternative privilégiée aux voies orales ou injectables.
- Cette tendance vers des thérapies topiques plus efficaces, plus conviviales pour les patients et technologiquement améliorées remodèle les attentes en matière de soins de santé à domicile et de traitement ambulatoire, stimulant l'innovation parmi les sociétés pharmaceutiques.
- La demande de systèmes d'administration topique de médicaments intégrant des techniques de pénétration avancées et des mécanismes de libération contrôlée augmente rapidement dans les milieux hospitaliers et de soins à domicile, car les patients privilégient la commodité, la sécurité et l'efficacité thérapeutique.
Dynamique du marché nord-américain de l'administration topique de médicaments
Conducteur
Besoin croissant en raison de l'augmentation des affections cutanées et des douleurs chroniques
- La prévalence croissante des troubles dermatologiques, des douleurs chroniques et des affections liées à l’âge est un facteur important de la demande accrue de systèmes d’administration topique de médicaments.
- Par exemple, l’augmentation des cas d’eczéma, de psoriasis et de douleurs musculo-squelettiques localisées incite les prestataires de soins de santé à adopter des options de traitement non invasives et ciblées.
- Les systèmes d'administration topique offrent une thérapie localisée, des effets secondaires systémiques réduits et une meilleure adhésion du patient par rapport aux formulations orales ou injectables, offrant ainsi une alternative de traitement convaincante.
- En outre, la sensibilisation croissante aux thérapies adaptées aux patients et la préférence pour l'auto-administration à domicile font de l'administration topique de médicaments un élément essentiel des protocoles de traitement dans les hôpitaux et les centres spécialisés.
- La commodité d’un dosage précis, la facilité d’application et l’intégration avec les appareils de soins de santé à domicile sont des facteurs clés qui propulsent l’adoption de l’administration topique de médicaments en Amérique du Nord, tandis que les prestataires de soins de santé continuent d’encourager son utilisation pour les maladies chroniques et dermatologiques.
Retenue/Défi
Problèmes d'irritation cutanée et obstacle à la conformité réglementaire
- Les préoccupations concernant l’irritation cutanée, les réactions allergiques et les effets indésirables locaux constituent un défi important pour une adoption plus large des produits d’administration topique de médicaments.
- Par exemple, certains patients souffrent d’érythème ou de dermatite en raison de l’utilisation prolongée de patchs transdermiques ou de certaines formulations chimiques, ce qui limite l’acceptation du produit.
- Répondre à ces préoccupations en matière de sécurité au moyen de matériaux biocompatibles, de formulations hypoallergéniques et de tests cliniques rigoureux est essentiel pour renforcer la confiance des patients et des prestataires de soins.
- En outre, le respect des normes réglementaires telles que les directives de la FDA pour les dispositifs et les formulations d’administration topique de médicaments ajoute de la complexité au développement des produits et à l’entrée sur le marché.
- Le coût relativement élevé des systèmes d’administration avancés par rapport aux crèmes ou gels topiques conventionnels peut également restreindre l’adoption, en particulier chez les patients sensibles au prix, ce qui nécessite un équilibre entre innovation, sécurité et abordabilité.
- Surmonter ces défis en améliorant la sécurité des produits, l’éducation des patients et des solutions rentables sera essentiel pour une croissance soutenue du marché nord-américain de l’administration de médicaments topiques.
Portée du marché nord-américain de l'administration topique de médicaments
Le marché est segmenté en fonction du type de produit, des techniques d’administration, du type thérapeutique, de la génération, du mode d’achat, de l’indication, de la voie d’administration, de l’utilisateur final et du canal de distribution.
- Par type de produit
En Amérique du Nord, le marché des formulations topiques d'administration de médicaments se segmente en fonction du type de produit : formulations et dispositifs d'administration. Le segment des formulations a dominé le marché, avec une part de chiffre d'affaires de 61,5 % en 2024, grâce à l'utilisation généralisée de crèmes, de gels et de patchs transdermiques pour les applications dermatologiques et la gestion de la douleur. Ces formulations sont plébiscitées pour leur praticité, leur facilité d'application et leur compatibilité avec un large éventail d'agents thérapeutiques. Les patients et les professionnels de santé privilégient souvent les formulations pour les traitements à domicile et les soins ambulatoires en raison de leur caractère non invasif. De plus, ces formulations offrent une flexibilité de dosage, une libération prolongée et une thérapie ciblée, améliorant ainsi l'observance du traitement par les patients. Les laboratoires pharmaceutiques continuent d'innover dans les technologies de formulation afin d'améliorer l'absorption, de minimiser les effets secondaires et d'accroître l'efficacité. La large disponibilité des formulations topiques sur ordonnance et en vente libre renforce encore la domination de ce segment.
Le segment des dispositifs d'administration de médicaments devrait connaître la croissance la plus rapide entre 2025 et 2032, stimulé par l'adoption croissante de systèmes d'administration avancés tels que les patchs à micro-aiguilles, les dispositifs d'ionophorèse et les systèmes transdermiques portables. Ces dispositifs améliorent la précision de l'administration des médicaments, optimisent les résultats thérapeutiques et soutiennent les plans de traitement chroniques à long terme. Ils sont de plus en plus intégrés aux technologies de santé intelligentes et aux systèmes de surveillance, permettant aux cliniciens et aux patients de suivre l'observance thérapeutique. La tendance croissante à la médecine personnalisée et à l'administration non invasive stimule encore davantage la demande. De plus, les autorisations réglementaires accordées à des dispositifs innovants aux États-Unis et au Canada accélèrent l'expansion du marché. Les prestataires de soins de santé et les hôpitaux investissent dans des dispositifs permettant une administration ciblée et un confort accru pour les patients, stimulant ainsi leur pénétration du marché.
- Par technique de livraison
En Amérique du Nord, le marché de l'administration topique de médicaments se segmente en fonction de la technique d'administration : ionophorèse, sonophorèse, ablation laser, ablation par radiofréquence, magnétophorèse, électroporation, etc. En 2024, l'ionophorèse dominait le marché avec une part de 28,9 %, grâce à sa capacité à améliorer de manière non invasive la pénétration des médicaments dans les couches cutanées ciblées. Largement utilisée pour les anti-inflammatoires et les analgésiques, l'ionophorèse offre un traitement localisé avec une exposition systémique réduite. Les patients bénéficient d'un délai d'action plus rapide et d'effets secondaires moins importants qu'avec l'administration orale. Les professionnels de santé privilégient souvent l'ionophorèse pour une administration contrôlée et une utilisation en ambulatoire. Les progrès en matière de conception de dispositifs et de patchs portables améliorent la commodité et l'observance du traitement. L'intégration de l'ionophorèse à des systèmes de surveillance intelligents permet un suivi de la dose et améliore les résultats thérapeutiques, soutenant ainsi une forte demande du marché.
Le segment de l'échographie devrait connaître la croissance la plus rapide entre 2025 et 2032, grâce à l'intensification des recherches sur l'administration transdermique assistée par ultrasons, qui améliore la perméabilité des grosses molécules et des vaccins. L'échographie est actuellement explorée pour l'administration non invasive d'hormones, d'analgésiques et de traitements dermatologiques. Son application en milieu hospitalier et à domicile se développe, les patients recherchant des options de traitement plus rapides et indolores. Les avancées technologiques et la réduction du coût des dispositifs favorisent son adoption. La sensibilisation croissante des dermatologues et des spécialistes de la gestion de la douleur à son efficacité devrait favoriser son adoption rapide.
- Par type
En Amérique du Nord, le marché des médicaments topiques est segmenté en agents nettoyants, agents protecteurs, agents hydratants, agents asséchants, agents anti-démangeaisons, agents anti-inflammatoires, agents anti-infectieux, kératolytiques, etc. Le segment des agents anti-inflammatoires dominait le marché avec une part de 33,2 % en 2024, en raison de la forte prévalence des inflammations cutanées chroniques, des douleurs musculo-squelettiques et des affections postopératoires en Amérique du Nord. Les produits anti-inflammatoires topiques sont privilégiés pour un soulagement localisé et minimisent les effets secondaires systémiques associés aux médicaments oraux. Ils sont couramment utilisés en dermatologie, en physiothérapie et en soins à domicile. La disponibilité de formulations sur ordonnance et en vente libre améliore l'accessibilité et l'observance du traitement par les patients. Les laboratoires pharmaceutiques continuent de développer des formulations anti-inflammatoires avancées offrant une meilleure absorption et des propriétés de libération prolongée. La sensibilisation croissante des patients et des cliniciens aux bienfaits des thérapies ciblées renforce la position de leader de ce segment sur le marché.
Le segment des agents hydratants devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par la demande croissante de produits dermatologiques cosmétiques et de soins préventifs. La sensibilisation accrue à l'hydratation cutanée, combinée à la prévalence de l'eczéma et des affections cutanées sèches, stimule l'adoption de ces produits. L'intégration des hydratants à d'autres agents thérapeutiques pour des thérapies combinées stimule leur utilisation. Le commerce en ligne et la vente au détail améliorent l'accessibilité, tandis que les innovations technologiques en matière de formulation améliorent la stabilité et l'efficacité. Cette croissance est également soutenue par le vieillissement de la population en quête de solutions de soins pour les peaux sensibles et matures.
- Par génération
En Amérique du Nord, le marché de l'administration topique de médicaments se segmente en systèmes d'administration transdermique de première, de deuxième et de troisième génération. Le segment des systèmes d'administration transdermique de deuxième génération dominait le marché avec une part de marché de 42,1 % en 2024, grâce à une meilleure perméabilité du médicament, un meilleur contrôle des taux de libération et une plus grande compatibilité avec divers médicaments. Les systèmes de deuxième génération, tels que les patchs d'ionophorèse et de sonophorèse, offrent un traitement localisé efficace, réduisent les effets secondaires systémiques et améliorent l'observance thérapeutique. Les professionnels de santé privilégient ces systèmes pour les affections chroniques nécessitant un dosage soutenu. Les laboratoires pharmaceutiques continuent d'investir dans les systèmes de deuxième génération en raison de leur équilibre entre efficacité, sécurité et fabricabilité. Leur succès clinique avéré, combiné à leur rentabilité par rapport aux systèmes de troisième génération, soutient une forte demande.
Le segment des systèmes d'administration transdermique de troisième génération devrait connaître la croissance la plus rapide entre 2025 et 2032, grâce aux innovations dans les réseaux de micro-aiguilles, l'administration assistée par nanovecteurs et les techniques transdermiques améliorées par laser. Ces systèmes permettent l'administration de molécules plus grosses, notamment des protéines et des vaccins, jusqu'alors inaccessibles à l'administration topique. Le développement de la recherche sur les formulations avancées et leur adoption croissante dans les hôpitaux spécialisés et les services de soins à domicile favorisent une adoption rapide. Les patients bénéficient d'une invasivité minimale et d'un dosage précis, ce qui favorise l'expansion du marché. Les autorisations réglementaires pour les systèmes de nouvelle génération accélèrent encore la croissance. Les essais cliniques démontrant l'innocuité et l'efficacité contribuent à des taux d'adoption élevés.
- Par mode d'achat
En Amérique du Nord, le marché des médicaments topiques est segmenté selon le mode d'achat : sur ordonnance et en vente libre (OTC). Le segment des médicaments sur ordonnance dominait le marché avec une part de 58,7 % en 2024, en raison de la prévalence des affections dermatologiques, des douleurs chroniques et d'autres indications thérapeutiques nécessitant une supervision professionnelle. Les produits sur ordonnance offrent un meilleur suivi de l'efficacité et de la sécurité, essentiel pour des médicaments tels que les anti-inflammatoires, les kératolytiques et les formulations topiques à base d'hormones. Les hôpitaux, les centres spécialisés et les cliniciens recommandent souvent des traitements topiques sur ordonnance pour de meilleurs résultats thérapeutiques. L'obligation d'approbation réglementaire garantit la qualité et la cohérence, ce qui renforce le segment des médicaments sur ordonnance. Les patients et les soignants privilégient également les conseils d'un professionnel de santé concernant la posologie, la durée et les traitements combinés, renforçant ainsi la domination de ce segment.
Le segment des médicaments en vente libre devrait connaître la croissance la plus rapide entre 2025 et 2032, grâce à la montée de l'automédication, à la sensibilisation croissante à la dermatologie préventive et à un accès facilité via les pharmacies de détail et en ligne. Les produits topiques en vente libre, tels que les crèmes hydratantes, les crèmes anti-démangeaisons et les agents protecteurs, sont de plus en plus utilisés pour les affections cutanées mineures et les soins à domicile. Le marketing numérique et le e-commerce améliorent leur visibilité et leur accessibilité. La préférence croissante des consommateurs pour des traitements pratiques et sans ordonnance soutient l'expansion du segment. Les entreprises innovent dans les formulations de médicaments en vente libre afin d'améliorer l'efficacité, la sécurité et l'esthétique.
- Par indication
En Amérique du Nord, le marché des médicaments topiques est segmenté, selon l'indication, en affections dermatologiques, ophtalmiques, gestion de la douleur, neurochirurgicales, hormonothérapie, sevrage tabagique, etc. Le segment des affections dermatologiques a dominé le marché avec une part de 44,3 % en 2024, porté par la forte incidence de l'acné, de l'eczéma, du psoriasis et d'autres affections cutanées en Amérique du Nord. Les formulations topiques offrent un traitement localisé avec moins d'effets secondaires systémiques que les médicaments oraux. Les hôpitaux, les cliniques de dermatologie et les services de soins à domicile adoptent largement ces traitements. L'innovation pharmaceutique dans les produits topiques anti-inflammatoires, anti-infectieux et hydratants renforce encore ce segment. La préférence des patients pour des traitements non invasifs et faciles à appliquer soutient une demande soutenue. La R&D continue visant à améliorer l'efficacité et l'observance du traitement contribue à cette domination.
Le segment de la gestion de la douleur devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par l'augmentation des cas de douleurs musculo-squelettiques chroniques, d'arthrite et d'inconfort postopératoire. Les analgésiques topiques, les gels et les patchs procurent un soulagement localisé et réduisent la dépendance aux analgésiques oraux. Leur adoption dans les hôpitaux, les centres spécialisés et les services de soins à domicile est en hausse. L'intégration de techniques d'administration avancées telles que l'ionophorèse accélère la croissance. Les campagnes de sensibilisation promouvant des alternatives plus sûres à la gestion systémique de la douleur soutiennent l'expansion du segment. Les améliorations technologiques en matière de formulation et de méthodes d'application conviviales pour le patient stimulent également l'adoption.
- Par voie d'administration
En Amérique du Nord, le marché de l'administration topique de médicaments se segmente en fonction de la voie d'administration : dermique, ophtalmique, rectale, vaginale, nasale, etc. Le segment dermique dominait le marché avec une part de 67,8 % en 2024, en raison de la forte demande pour le traitement localisé des affections cutanées et la gestion de la douleur. L'administration dermique permet un ciblage précis, minimise l'absorption systémique et améliore le confort du patient. Les professionnels de santé et les patients recevant des soins à domicile privilégient l'administration dermique pour sa facilité d'utilisation, son caractère non invasif et sa compatibilité avec les patchs et gels avancés. Les innovations constantes en matière d'activateurs de pénétration, d'ionophorèse et de systèmes à libération prolongée renforcent ce segment. Les produits dermiques sur ordonnance et en vente libre dominent les marchés des hôpitaux, des pharmacies et des soins à domicile. Les recherches continues sur l'amélioration de l'absorption dermique soutiennent une croissance soutenue.
Le segment ophtalmique devrait connaître la croissance la plus rapide entre 2025 et 2032, portée par la prévalence croissante des troubles oculaires, l'augmentation de la population âgée et les innovations en matière de systèmes d'administration oculaire non invasifs de médicaments. Gouttes, gels et inserts deviennent des options privilégiées pour les traitements localisés, améliorant ainsi les résultats thérapeutiques. Les autorisations réglementaires et les validations cliniques renforcent la confiance du marché. L'expansion dans les hôpitaux, les centres spécialisés et les soins à domicile favorise l'adoption. Les avancées technologiques en matière de formulation améliorent la rétention et l'efficacité des médicaments. Les plateformes de santé numérique favorisant l'observance accélèrent la croissance.
- Par utilisateur final
En Amérique du Nord, le marché de l'administration topique de médicaments est segmenté selon l'utilisateur final : hôpitaux, centres spécialisés, soins à domicile, etc. Le segment hospitalier a dominé le marché avec une part de 49,2 % en 2024, grâce à l'adoption massive des traitements topiques pour les soins dermatologiques, la gestion de la douleur et les soins postopératoires. Les hôpitaux assurent une supervision professionnelle, garantissant la bonne utilisation, l'efficacité et la sécurité des systèmes d'administration topique. Ils prennent également en charge des techniques d'administration avancées telles que l'ionophorèse et les patchs à micro-aiguilles. La forte fréquentation des établissements et l'intégration avec les services pharmaceutiques renforcent la domination des hôpitaux. Ces derniers continuent d'adopter des produits innovants pour améliorer les résultats des traitements et la satisfaction des patients. Les collaborations de recherche entre hôpitaux et laboratoires pharmaceutiques favorisent l'adoption de traitements topiques de nouvelle génération.
Le segment des soins à domicile devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par la préférence croissante pour les thérapies auto-administrées, la prise en charge des maladies chroniques et l'intégration de la télésanté. Les patients ont de plus en plus recours aux thérapies topiques à domicile pour des raisons de commodité, d'économies et de continuité des soins. Cette croissance est soutenue par des dispositifs d'administration portables et intelligents. Les initiatives éducatives et les programmes de sensibilisation des patients accélèrent l'adoption. L'intégration aux plateformes de télésurveillance et de santé numérique améliore l'observance et la sécurité. L'expansion des services de soins à domicile en Amérique du Nord stimule la pénétration du marché.
- Par canal de distribution
En Amérique du Nord, le marché des médicaments topiques est segmenté selon les canaux de distribution : pharmacies hospitalières, pharmacies de détail, pharmacies en ligne, etc. En 2024, le segment des pharmacies de détail dominait le marché avec une part de 52,5 %, grâce à la facilité d'accès aux produits topiques sur ordonnance et en vente libre. Les pharmacies de détail offrent à la fois un accompagnement professionnel et une disponibilité immédiate, ce qui en fait le choix privilégié des patients. Elles proposent également des programmes de fidélité, des remises promotionnelles et des services de conseil, renforçant ainsi l'engagement client. La présence de nombreux produits topiques de marque et génériques dans les points de vente renforce encore ce segment. De solides réseaux de distribution et des partenariats avec des laboratoires pharmaceutiques renforcent la domination des pharmacies de détail. L'expansion continue des chaînes de distribution en zones urbaines et semi-urbaines soutient une croissance continue.
Le segment des pharmacies en ligne devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par la pénétration croissante du e-commerce, la préférence croissante pour la livraison à domicile et l'adoption de la santé numérique. Les pharmacies en ligne offrent commodité, services d'abonnement et une gamme de produits plus large, attirant ainsi les consommateurs férus de technologie. La facilité d'accès pendant et après la pandémie a accéléré l'adoption. Les plateformes numériques offrent également l'intégration de la téléconsultation et de la livraison à domicile. La confiance croissante des consommateurs et les stratégies promotionnelles des laboratoires pharmaceutiques stimulent l'expansion du marché.
Analyse régionale du marché nord-américain de l'administration topique de médicaments
- Les États-Unis ont dominé le marché nord-américain de l'administration topique de médicaments avec la plus grande part de revenus de 85,3 % en 2024, caractérisé par une infrastructure de recherche pharmaceutique avancée, des dépenses de santé élevées et une adoption généralisée de technologies innovantes d'administration de médicaments, avec une croissance substantielle des patchs et gels transdermiques tirée par les progrès des systèmes d'administration transdermique de deuxième génération.
- Les patients et les prestataires de soins de santé de la région privilégient la commodité, la thérapie ciblée et la réduction des effets secondaires systémiques, ce qui fait que les systèmes d'administration topique de médicaments sont de plus en plus préférés aux formulations orales ou injectables.
- Cette adoption généralisée est en outre soutenue par une infrastructure de soins de santé avancée, de solides capacités de recherche et développement pharmaceutiques, des dépenses de santé élevées et une sensibilisation croissante aux solutions de soins de santé à domicile, établissant l'administration topique de médicaments comme une option de traitement privilégiée dans les hôpitaux, les centres spécialisés et les établissements de soins de santé à domicile.
Aperçu du marché nord-américain de l'administration topique de médicaments
Le marché américain de l'administration topique de médicaments a représenté la plus grande part de chiffre d'affaires en 2024 en Amérique du Nord, grâce à la forte prévalence des affections dermatologiques et des douleurs chroniques, ainsi qu'à l'adoption croissante de techniques d'administration non invasives avancées telles que l'ionophorèse et la sonophorèse. Patients et professionnels de santé privilégient de plus en plus les thérapies ciblées et localisées afin de réduire les effets secondaires systémiques et d'améliorer l'observance thérapeutique. La préférence croissante pour les thérapies à domicile, combinée à l'intégration de la surveillance médicale numérique et des dispositifs portables d'administration de médicaments, stimule encore davantage le marché. De plus, la présence d'une solide infrastructure de recherche et développement pharmaceutique, les dépenses de santé élevées et le soutien réglementaire aux systèmes d'administration innovants contribuent significativement à l'expansion du marché.
Aperçu du marché canadien de l'administration topique de médicaments
Le marché canadien de l'administration topique de médicaments devrait connaître une croissance substantielle au cours de la période de prévision, principalement grâce à une sensibilisation accrue à la santé cutanée, à la gestion de la douleur chronique et aux traitements préventifs. L'urbanisation croissante et la hausse des revenus disponibles favorisent l'adoption de formulations topiques et de dispositifs d'administration avancés. Les patients canadiens sont également attirés par la commodité, la sécurité et la convivialité offertes par les solutions d'administration non invasive de médicaments. La région connaît une croissance dans les hôpitaux, les centres spécialisés et les applications de soins à domicile, avec une adoption croissante des produits topiques, qu'ils soient sur ordonnance ou en vente libre. Les initiatives gouvernementales favorisant l'éducation des patients et l'intégration de la télésanté contribuent également à l'expansion du marché.
Aperçu du marché mexicain de l'administration de médicaments topiques
Le marché mexicain de l'administration topique de médicaments devrait connaître une croissance significative au cours de la période de prévision, portée par la prévalence croissante des troubles dermatologiques et musculo-squelettiques et par la demande croissante de solutions thérapeutiques pratiques à domicile. La sensibilisation croissante aux soins de santé et l'adoption de techniques modernes d'administration de médicaments encouragent les patients et les professionnels de santé à privilégier les traitements localisés aux médicaments systémiques. La disponibilité de produits topiques abordables et la pénétration croissante des pharmacies de détail et en ligne stimulent la croissance du marché. Le développement des infrastructures de santé mexicaines et les partenariats avec des multinationales pharmaceutiques améliorent l'accessibilité et l'adoption de formulations topiques avancées. La tendance vers des traitements non invasifs et conviviaux pour les patients devrait continuer à stimuler la croissance du marché.
Part de marché des médicaments topiques en Amérique du Nord
L’industrie nord-américaine de l’administration de médicaments topiques est principalement dirigée par des entreprises bien établies, notamment :
- 3M (États-Unis)
- AbbVie Inc. (États-Unis)
- Cipla (Inde)
- Galderma SA (Suisse)
- Hisamitsu Pharmaceutical Co., Inc. (Japon)
- Johnson & Johnson et ses filiales (États-Unis)
- Merck & Co., Inc. (États-Unis)
- Novartis AG (Suisse)
- Piramal Pharma Solutions (Inde)
- Teva Pharmaceuticals (Israël)
- Bausch Health Companies Inc. (Canada)
- Lupin (Inde)
- Amgen Inc. (États-Unis)
- Sandoz Inc. (États-Unis)
- Dr. Reddy's Laboratories Ltd. (Inde)
- Sun Pharmaceutical Industries Ltd. (Inde)
- Zydus Cadila (Inde)
- Boehringer Ingelheim Pharmaceuticals, Inc. (Allemagne)
- Sanofi (France)
Quels sont les développements récents sur le marché nord-américain de l’administration de médicaments topiques ?
- En novembre 2024, Endo, Inc. a annoncé que sa filiale, Paladin Pharma Inc., avait conclu une entente définitive avec MC2 Therapeutics pour la commercialisation de la crème Wynzora au Canada. Si elle est approuvée par Santé Canada, cette collaboration offrira aux patients canadiens une nouvelle option de traitement contre le psoriasis en plaques, tirant parti de l'expérience de Paladin en dermatologie et de la formulation innovante de MC2.
- En mai 2024, Formosa Pharmaceuticals (« Formosa », 6838.TWO), basée à Taïwan, a annoncé que la société avait conclu un accord de licence exclusif avec Tabuk Pharmaceuticals Manufacturing Company (« Tabuk »), pour les droits exclusifs de commercialisation de la suspension ophtalmique de propionate de clobétasol, 0,05 % (APP13007), un médicament innovant breveté pour le traitement de l'inflammation et de la douleur après une chirurgie oculaire.
- En mars 2024, Formosa Pharmaceuticals a annoncé que la FDA américaine avait approuvé l'APP13007, une suspension ophtalmique de propionate de clobétasol (0,05 %), pour le traitement de l'inflammation et de la douleur postopératoires. Cette approbation marque une avancée significative dans les soins postopératoires des patients opérés des yeux.
- En août 2023, la FDA américaine a émis un avertissement public concernant les préparations topiques à base de finastéride commercialisées pour le traitement de la chute des cheveux. L'agence a souligné l'absence de préparations topiques approuvées par la FDA et recensé 32 rapports d'effets indésirables associés à ces produits non approuvés entre 2019 et 2024.
- En février 2021, Almirall, société biopharmaceutique mondiale, a conclu un accord de licence, de collaboration et de commercialisation avec MC2 Therapeutics, lui accordant ainsi les droits exclusifs de commercialisation en Europe de la crème Wynzora (calcipotriène et dipropionate de bétaméthasone) pour le traitement du psoriasis en plaques. Aux termes de cet accord, MC2 Therapeutics est responsable de la fabrication et de l'approvisionnement, tandis qu'Almirall se concentre sur la commercialisation en Europe.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.