North America Prophylaxis Of Organ Rejection Market
Taille du marché en milliards USD
TCAC :
% 
 USD
1,092.43 Million
USD
1,560.26 Million
2021
2029
USD
1,092.43 Million
USD
1,560.26 Million
2021
2029
| 2022 –2029 | |
| USD 1,092.43 Million | |
| USD 1,560.26 Million | |
|
|
|
Marché nord-américain de la prophylaxie du rejet d'organes, par cause (exposition épidémiologique, prophylaxie antibactérienne, prophylaxie contre d'autres agents pathogènes et autres), traitement (immunosuppresseur ambulatoire, immunosuppresseur hospitalier et autres), voie d'administration (orale, intraveineuse), organe (rein, foie, cœur, poumon, autres), type de patient (pédiatrie, adultes), utilisateur final (hôpitaux, cliniques de soins à domicile et autres), canal de distribution (appel d'offres direct, pharmacies et autres), pays (États-Unis, Canada, Mexique), tendances du marché et prévisions jusqu'en 2029.
Analyse et perspectives du marché : Marché nord-américain de la prophylaxie du rejet d'organes
Le marché nord-américain de la prophylaxie du rejet d’organes devrait connaître une croissance du marché au cours de la période de prévision de 2022 à 2029. Data Bridge Market Research analyse que le marché croît avec un TCAC de 4,7 % au cours de la période de prévision de 2022 à 2029 et devrait atteindre 1 560,26 millions USD d’ici 2029 contre 1 092,43 millions USD en 2021. La prévalence croissante de la transplantation d’organes et l’utilisation accrue d’immunosuppresseurs sont susceptibles d’être les principaux moteurs de la demande du marché au cours de la période de prévision.
Le terme prophylaxie désigne un traitement administré ou une action entreprise pour prévenir une maladie. La prophylaxie du rejet d'organe fait référence à la prévention du rejet d'organe par l'utilisation de médicaments. Les patients qui subissent une transplantation doivent être maintenus sous un régime d'immunosuppression pour la prophylaxie du rejet afin de garantir la survie du greffon. Le rejet de greffe est un processus dans lequel l'immunité du receveur de greffe est affaiblie par l'utilisation d'inhibiteurs de la calcineurine, d'inhibiteurs de mTOR, d'agents antimétabolites et de corticostéroïdes. Les agents de traitement améliorent les conséquences à court terme de la transplantation d'organe, mais certaines améliorations sont possibles. Les patients receveurs, qui ont subi une transplantation d'organe solide, doivent prendre des médicaments anti-rejet. En effet, le système immunitaire détruirait l'organe transplanté.
Les facteurs responsables de la croissance du marché nord-américain de la prophylaxie du rejet d'organes sont la prévalence accrue de la transplantation d'organes, l'augmentation des interventions chirurgicales, l'augmentation de la population de receveurs et le lancement de nouveaux produits. Cependant, les facteurs susceptibles de freiner le marché sont l'augmentation du coût de la procédure de transplantation, le manque de sensibilisation à la transplantation d'organes et les risques encourus par le patient lors de la réception de l'organe transplanté.
- D'autre part, les initiatives stratégiques des acteurs du marché, l'augmentation de la recherche et du développement et l'utilisation d'immunosuppresseurs peuvent constituer une opportunité pour la croissance du marché nord-américain de la prophylaxie du rejet d'organes. Le besoin d'expertise qualifiée et l'approbation réglementaire peuvent créer des défis pour le marché nord-américain de la prophylaxie du rejet d'organes. Des développements récents liés au marché nord-américain de la prophylaxie du rejet d'organes ont été observés.
Le rapport sur le marché nord-américain de la prophylaxie du rejet d'organes fournit des détails sur la part de marché, les nouveaux développements et l'impact des acteurs du marché national et local, analyse les opportunités en termes de poches de revenus émergentes, de changements dans la réglementation du marché, d'approbations de produits, de décisions stratégiques, de lancements de produits, d'expansions géographiques et d'innovations technologiques sur le marché. Pour comprendre l'analyse et le scénario du marché, contactez-nous pour un briefing d'analyste, notre équipe vous aidera à créer une solution d'impact sur les revenus pour atteindre votre objectif souhaité.
Portée et taille du marché de la prophylaxie du rejet d'organes en Amérique du Nord
Le marché nord-américain de la prophylaxie du rejet d’organes est classé en sept segments : cause, traitement, voie d’administration, organe, type de patient, utilisateur final et canal de distribution.
La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d’application et la différence dans vos marchés cibles.
- Sur la base de la cause, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en exposition épidémiologique, prophylaxie antibactérienne, prophylaxie contre d'autres agents pathogènes et autres. En 2022, le segment de l'exposition épidémiologique devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes en raison de la disponibilité d'informations directes et de la présence d'agents pathogènes liés à la prophylaxie du rejet d'organes aux États-Unis
- En fonction du traitement, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en immunosuppresseurs ambulatoires, immunosuppresseurs hospitaliers et autres. En 2022, le segment des immunosuppresseurs ambulatoires devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes, car il permet d'obtenir une réponse immunitaire soutenue et spécifique contre les cellules endommagées et la disponibilité de la cyclosporine aux États-Unis et au Canada.
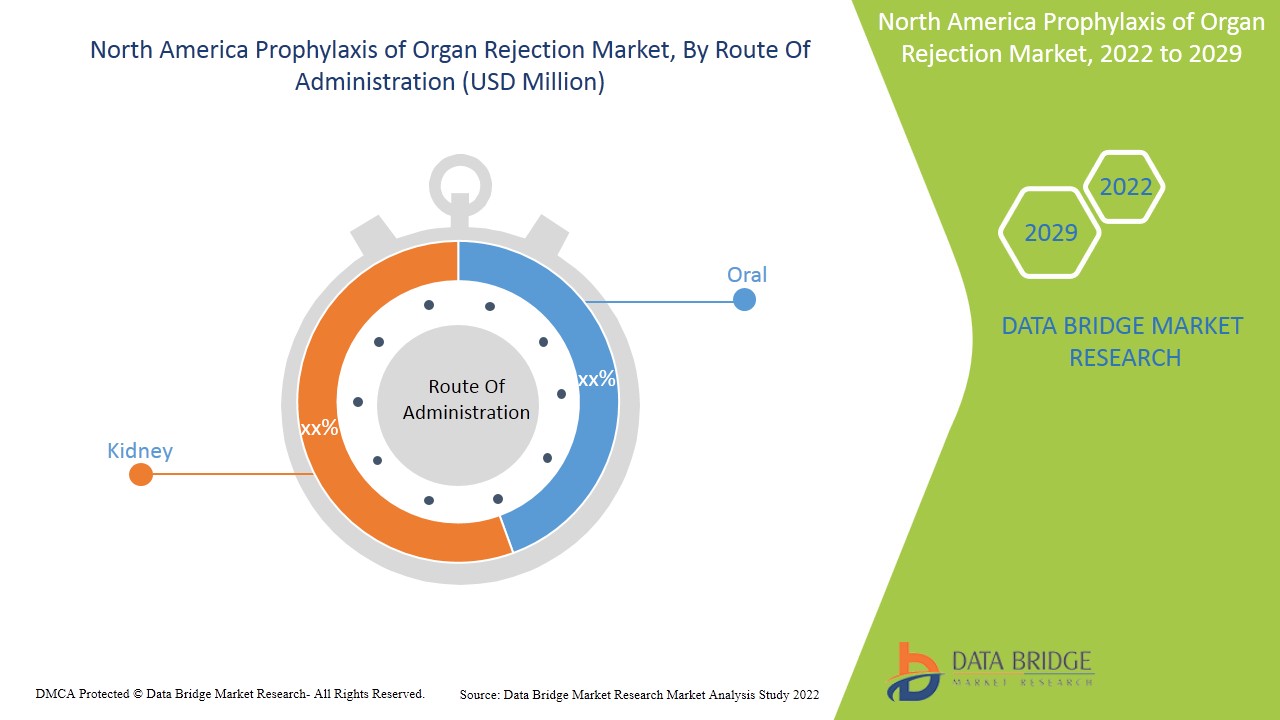
- En fonction de la voie d'administration, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en voie orale et intraveineuse . En 2022, le segment oral devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes en raison de la disponibilité et de la consommation accrues de comprimés et de gélules oraux.
- Sur la base des organes, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en rein, foie, cœur, poumon et autres. En 2022, le segment rénal devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes en raison de l'incidence accrue de l'insuffisance rénale et de la présence de politiques de remboursement telles que Medicare, pour l'assurance du receveur.
- En fonction du type de patient, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en pédiatrie et en adultes. En 2022, le segment des adultes devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes, en raison de la prévalence accrue des maladies chroniques et des conditions génétiques des patients adultes, de la faiblesse du système immunitaire chez les adultes et de la liste d'attente des receveurs adultes supérieure à la liste d'attente des enfants et des femmes au Nouveau-Mexique et aux États-Unis
- En fonction de l'utilisateur final, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en hôpitaux, cliniques, soins à domicile et autres. En 2022, le segment des hôpitaux devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes en raison de l'augmentation des soins médicaux et de la collaboration avec d'autres hôpitaux en Amérique du Nord pour la livraison des organes des donneurs aux patients receveurs.
- En fonction du canal de distribution, le marché nord-américain de la prophylaxie du rejet d'organes est segmenté en appels d'offres directs, pharmacies et autres. En 2022, le segment des appels d'offres directs devrait dominer le marché nord-américain de la prophylaxie du rejet d'organes en raison de la forte demande d'immunosuppresseurs ambulatoires par les sociétés pharmaceutiques et du paiement garanti, qui devraient dominer le marché.
Analyse du marché de la prophylaxie du rejet d'organes en Amérique du Nord
Le marché nord-américain de la prophylaxie du rejet d’organes est classé en sept segments : cause, traitement, voie d’administration, organe, type de patient, utilisateur final et canal de distribution.
Les pays couverts par le rapport sur le marché de la prophylaxie du rejet d’organes en Amérique du Nord sont les États-Unis, le Canada et le Mexique.
- En 2021, les États-Unis dominent le marché en raison de la présence du plus grand marché de consommation avec un PIB élevé. De plus, les États-Unis ont les dépenses des ménages les plus élevées au monde et ont conclu des accords commerciaux avec plusieurs pays, ce qui en fait le plus grand marché pour les produits de consommation, y compris les produits destinés à la prophylaxie du rejet d'organes, en raison de la présence d'acteurs majeurs du marché et des progrès technologiques accrus dans la région.
La section du rapport sur les pays fournit également des facteurs d'impact sur les marchés individuels et des changements de réglementation sur le marché national qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie des pays, les actes réglementaires et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario du marché pour les différents pays. En outre, la présence et la disponibilité des marques nord-américaines et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des canaux de vente sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Le potentiel de croissance de la prophylaxie du rejet d'organes dans les économies émergentes et les initiatives stratégiques des acteurs du marché créent de nouvelles opportunités sur le marché nord-américain de la prophylaxie du rejet d'organes
Le marché nord-américain de la prophylaxie du rejet d'organes vous fournit également une analyse de marché détaillée pour la croissance de chaque pays dans un secteur particulier avec les ventes de prophylaxie du rejet d'organes. L'impact des progrès dans la prophylaxie du rejet d'organes et les changements dans les scénarios réglementaires avec leur soutien au marché de la prophylaxie du rejet d'organes. Les données sont disponibles pour la période historique de 2020 à 2021.
Analyse du paysage concurrentiel et des parts de marché de la prophylaxie du rejet d'organes en Amérique du Nord
Le paysage concurrentiel du marché de la prophylaxie du rejet d'organes en Amérique du Nord fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, les sites et installations de production, les forces et les faiblesses de l'entreprise, le lancement de produits, les pipelines d'essais de produits, les approbations de produits, les brevets, la largeur et l'ampleur du produit, la domination des applications, la courbe de survie technologique. Les points de données ci-dessus fournis ne concernent que l'orientation de l'entreprise par rapport au marché de la prophylaxie du rejet d'organes en Amérique du Nord.
Français Les principales entreprises fournissant la prophylaxie du rejet d'organes sont Ossium Health, Inc, Altavant Sciences, Inc, Concord Biotech, WOCKHARDT, SEBELA PHARMACEUTICALS, Veloxis Pharmaceuticals, Inc, Astellas Pharma Inc, Novartis AG, Bristol-Myers Squibb Company, Dr Reddy's Laboratories, Ltd, Viatris Inc, Strides Pharma Science Limited, Glenmark, Biocon, Pfizer Inc, Mayne Pharma Group Limited, Hikma Pharmaceuticals PLC, AbbVie Inc, Apotex Inc, Teva Pharmaceutical USA Inc, Amneal Pharmaceuticals LLC, Zydus Pharmaceuticals, Inc., Novitium Pharma, ZHEJIANG HISUN PHARMACEUTICAL Co., LTD., CSL Behring entre autres.
Les analystes DBMR comprennent les atouts concurrentiels et fournissent une analyse concurrentielle pour chaque concurrent séparément.
Les initiatives stratégiques des acteurs du marché ainsi que les nouvelles avancées technologiques pour la prophylaxie du rejet d’organes en Amérique du Nord comblent le fossé dans l’utilisation de médicaments pour éviter le rejet d’organes.
Par exemple,
- En janvier 2020, Altavant Sciences, Inc. a acquis Onspira Therapeutics. Altavant Sciences Inc. avait développé l'OSP-101 pour le traitement du syndrome de bronchiolite oblitérante, principale cause de morbidité et de mortalité chez les patients ayant subi une transplantation pulmonaire. L'acquisition élargirait le portefeuille de produits et inclurait l'OSP-101, en vue d'une approbation post-commercialisation.
La collaboration, les coentreprises et d’autres stratégies des acteurs du marché améliorent le marché de l’entreprise sur le marché de la prophylaxie du rejet d’organes, ce qui offre également l’avantage à l’organisation d’améliorer son offre pour le marché nord-américain de la prophylaxie du rejet d’organes.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 BY CAUSE SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: EPIDEMIOLOGY SNAPSHOT
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN PREVALENCE OF ORGAN TRANSPLANTATIONS
5.1.2 RISE IN CHRONIC DISEASES
5.1.3 RISE IN CLINICAL TRIALS
5.1.4 RISE IN RECIPIENT PATIENTS
5.1.5 PRODUCT APPROVALS
5.2 RESTRAINTS
5.2.1 RISE IN COST OF TRANSPLANTATION PROCEDURE
5.2.2 LACK OF AWARENESS ABOUT ORGAN TRANSPLANTATION
5.2.3 RISKS INCLUDED WITH THE PATIENT RECEIVES TRANSPLANTED ORGAN
5.2.4 ETHICAL ISSUES RELATED TO ORGAN TRANSPLANTATION
5.3 OPPORTUNITIES
5.3.1 RISE IN RESEARCH AND DEVELOPMENTS
5.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
5.3.3 RISE IN USE OF IMMUNOSUPPRESSANT
5.4 CHALLENGES
5.4.1 SHORTAGE OF SKILLED PROFESSIONALS REQUIRED FOR THE USE OF PROPHYLAXIS OF ORGAN REJECTION
5.4.2 STRINGENT REGULATIONS
6 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE
6.1 OVERVIEW
6.2 EPIDEMIOLOGIC EXPOSURE
6.2.1 HOSPITAL EXPOSURE
6.2.2 COMMUNITY EXPOSURE
6.3 ANTIBACTERIAL PROPHYLAXIS
6.3.1 POST-TRANSPLANT PROPHYLAXIS
6.3.2 PERIOPERATIVE ANTIBACTERIAL PROPHYLAXIS
6.4 PROPHYLAXIS AGAINST OTHER PATHOGENS
6.5 OTHERS
7 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT
7.1 OVERVIEW
7.2 OUTPATIENT IMMUNOSUPPRESSANT
7.2.1 CALCINEURIN INHIBITORS
7.2.1.1 CYCLOSPORINE
7.2.1.2 TACROLIMUS
7.2.1.2.1 IMMEDIATE RELEASE
7.2.1.2.2 EXTENDED RELEASE
7.2.2 CORTICOSTEROIDS
7.2.2.1 METHYLPREDNISOLONE
7.2.2.2 PREDNISONE
7.2.2.3 OTHERS
7.2.3 MYCOPHENOLIC ACIDS
7.2.3.1 MYCOPHENOLATE MOFETIL
7.2.3.2 MYCOPHENOLATE SODIUM
7.2.4 MTOR INHIBITORS
7.2.4.1 SIROLIMUS
7.2.4.2 EVEROLIMUS
7.2.5 AZATHIOPRINE
7.2.6 OTHERS
7.3 INPATIENT IMMUNOSUPPRESSANT
7.3.1 BELATACEPT
7.3.2 BASILIXIMAB
7.3.3 OTHERS
7.4 OTHERS
7.4.1 ERYTHROPOIESIS-STIMULATING AGENTS
7.4.1.1 ERYTHROPOIETIN
7.4.1.2 DARBEPOETIN
7.4.2 ANTI-VIRAL AGENTS
7.4.2.1 VALGANCICLOVIR
7.4.2.2 LAMIVUDINE
7.4.2.3 ADEFOVIR
7.4.2.4 OTHERS
7.4.3 OTHERS
8 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION
8.1 OVERVIEW
8.2 ORAL
8.2.1 TABLET
8.2.1.1 IMMEDIATE RELEASE
8.2.1.2 EXTENDED-RELEASE
8.2.2 ORAL SOLUTION
8.2.3 OTHERS
8.3 INTRAVENOUS
9 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ORGAN
9.1 OVERVIEW
9.2 KIDNEY
9.3 LIVER
9.4 HEART
9.5 LUNG
9.6 OTHERS
10 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 ADULTS
10.3 PEDIATRIC
11 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 HOME HEALTHCARE
11.5 OTHERS
12 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDERS
12.3 PHARMACY STORES
12.3.1 HOSPITAL PHARMACY
12.3.2 RETAIL PHARMACY
12.3.3 ONLINE PHARMACY
12.4 OTHERS
13 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 QUESTIONNAIRE
16 RELATED REPORTS
Liste des tableaux
TABLE 1 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA PROPHYLAXIS AGAINST OTHER PATHOGENS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA ORAL IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA ORAL IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA TABLET IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA INTRAVENOUS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA KIDNEY IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA LIVER IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA HEART IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA LUNG IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA ADULTS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA PEDIATRIC IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA HOSPITALS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA CLINICS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA HOME HEALTHCARE IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA DIRECT TENDERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 56 NORTH AMERICA CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 57 NORTH AMERICA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 58 NORTH AMERICA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 59 NORTH AMERICA ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 60 NORTH AMERICA ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 61 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 62 NORTH AMERICA ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 63 NORTH AMERICA TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 64 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 65 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 66 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 67 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 68 NORTH AMERICA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 69 U.S. PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 70 U.S. EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 71 U.S. ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 72 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 73 U.S. OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 74 U.S. CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 75 U.S. TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 76 U.S. MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 77 U.S. MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 78 U.S. CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 79 U.S. INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 80 U.S. OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 81 U.S. ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 82 U.S. ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 83 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 84 U.S. ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 85 U.S. TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 86 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 87 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 88 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 89 U.S. PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 90 U.S. PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 91 CANADA PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 92 CANADA EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 93 CANADA ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 94 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 95 CANADA OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 96 CANADA CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 97 CANADA TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 98 CANADA MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 99 CANADA MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 100 CANADA CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 101 CANADA INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 102 CANADA OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 103 CANADA ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 104 CANADA ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 105 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 106 CANADA ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 107 CANADA TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 108 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 109 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 110 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 111 CANADA PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 112 CANADA PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 113 MEXICO PROPHYLAXIS OF ORGAN TRANSPLANT, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 114 MEXICO EPIDEMIOLOGIC EXPOSURE IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 115 MEXICO ANTIBACTERIAL PROPHYLAXIS IN PROPHYLAXIS OF ORGAN REJECTION, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 116 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 117 MEXICO OUTPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 118 MEXICO CALCINEURIN INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 119 MEXICO TACROLIMUS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 120 MEXICO MYCOPHENOLIC ACIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 121 MEXICO MTOR INHIBITORS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 122 MEXICO CORTICOSTEROIDS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 123 MEXICO INPATIENT IMMUNOSUPPRESSANT IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 124 MEXICO OTHERS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 125 MEXICO ERYTHROPOIESIS-STIMULATING AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 126 MEXICO ANTI-VIRAL AGENTS IN PROPHYLAXIS OF ORGAN REJECTION, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 127 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 128 MEXICO ORAL IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 129 MEXICO TABLET IN PROPHYLAXIS OF ORGAN REJECTION, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 130 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY ORGAN, 2020-2029 (USD MILLION)
TABLE 131 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 132 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY END USER, 2020-2029 (USD MILLION)
TABLE 133 MEXICO PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 134 MEXICO PHARMACY STORES IN PROPHYLAXIS OF ORGAN REJECTION, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: DBMR POSITION GRID
FIGURE 8 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED PREVALENCE OF ORGAN TRANSPLANTATION, RISE IN RECIPIENT PATIENTS, AND PRODUCT APPPROVALS ARE EXPECTED TO DRIVE NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET FROM 2022 TO 2029
FIGURE 13 EPIDEMIOLOGIC EXPOSURE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET FROM 2022 & 2029
FIGURE 14 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR PROPHYLAXIS OF ORGAN REJECTION MANUFACTURERS IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 15 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: INCIDENCE, 2021
FIGURE 16 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: INCIDENCE REGIONAL LEVEL, 2021
FIGURE 17 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: PREVALENCE (2018-2021) AT REGIONAL LEVEL SNAPSHOT
FIGURE 18 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION: SURVIVAL OF PATIENT SNAPSHOT
FIGURE 19 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET
FIGURE 20 INCREASE COUNT OF KIDNEY TRANSPLANTATIONS IN THE U.S., 2020
FIGURE 21 TOTAL NUMBER OF ORGAN TRANSPLANTATION IN THE NORTH AMERICA SCENARIO, 2020
FIGURE 22 TOTAL NUMBER OF PATIENTS ON WAITING LIST VS TRANSPLANTS PERFORMED BY ORGAN IN 2020
FIGURE 23 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, 2021
FIGURE 24 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, 2020-2029 (USD MILLION)
FIGURE 25 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, CAGR (2022-2029)
FIGURE 26 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 27 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, 2021
FIGURE 28 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, 2020-2029 (USD MILLION)
FIGURE 29 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, CAGR (2022-2029)
FIGURE 30 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 31 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 32 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
FIGURE 33 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 34 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 35 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, 2021
FIGURE 36 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, 2020-2029 (USD MILLION)
FIGURE 37 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, CAGR (2022-2029)
FIGURE 38 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY ORGAN, LIFELINE CURVE
FIGURE 39 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, 2021
FIGURE 40 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, 2020-2029 (USD MILLION)
FIGURE 41 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 42 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 43 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, 2021
FIGURE 44 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 45 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, CAGR (2022-2029)
FIGURE 46 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY END USER, LIFELINE CURVE
FIGURE 47 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 48 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 49 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 50 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 51 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: SNAPSHOT (2021)
FIGURE 52 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY COUNTRY (2021)
FIGURE 53 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY COUNTRY (2022 & 2029)
FIGURE 54 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY COUNTRY (2021 & 2029)
FIGURE 55 NORTH AMERICA PROPHYLAXIS OF ORGAN TRANSPLANT MARKET: BY CAUSE (2022-2029)
FIGURE 56 NORTH AMERICA PROPHYLAXIS OF ORGAN REJECTION MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.