Marché nord-américain du diagnostic de la leucémie myéloïde aiguë, par type de produit (instruments et consommables et accessoires), type de test (test d'imagerie, test sanguin, tests de moelle osseuse, test de biomarqueurs, immunophénotypage, tests génétiques et autres), type de cancer (myéloblastique (M0), myéloblastique (M1), myéloblastique (M2), promyélocytaire (M3), myélomonocytaire (M4), monocytaire (M5), érythroleucémie (M6) et mégacaryocytaire (M7)), groupe d'âge (moins de 21 ans, 21-29, 30-65 ans et 65 ans et plus), sexe (homme et femme), utilisateur final (hôpital, laboratoires associés, laboratoires de diagnostic indépendants, centres d'imagerie diagnostique, instituts de recherche sur le cancer et autres), canal de distribution (appel d'offres direct et vente au détail) Tendances de l'industrie et prévisions jusqu'en 2030.
Analyse et perspectives du marché du diagnostic de la leucémie myéloïde aiguë en Amérique du Nord
En raison de l'augmentation des dépenses de santé, la croissance du marché s'accélère, ce qui peut se traduire par de meilleures opportunités de recherche et développement. En outre, l'augmentation du nombre de laboratoires de recherche clinique et de diagnostic pour le diagnostic du cancer permet au marché de se développer.
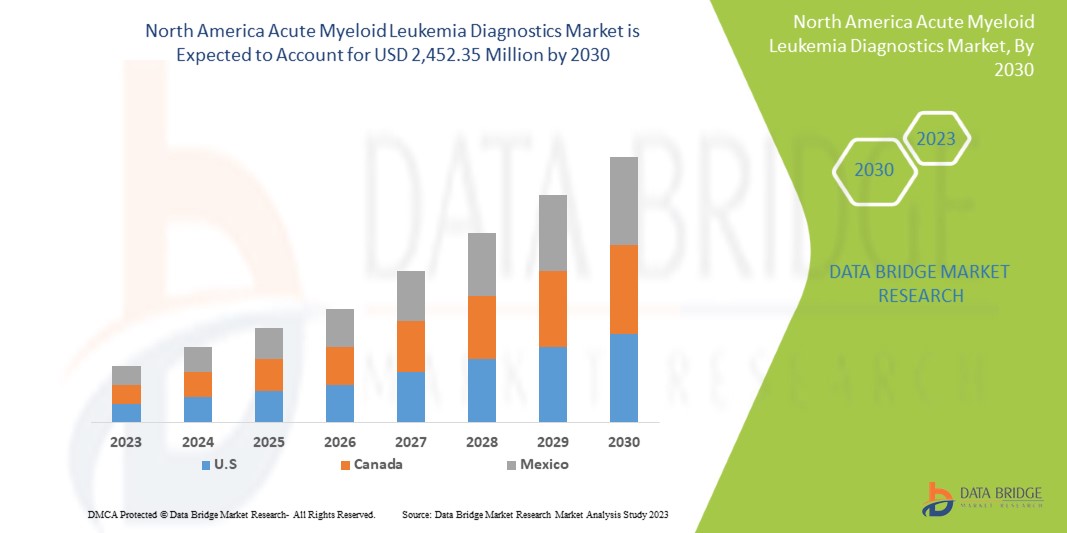
Le marché nord-américain du diagnostic de la leucémie myéloïde aiguë devrait croître au cours de la période de prévision de 2023 à 2030. Data Bridge Market Research analyse que le marché croît avec un TCAC de 11,7 % au cours de la période de prévision de 2023 à 2030 et devrait atteindre 2 452,35 millions USD d'ici 2030.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Année historique |
2021 (personnalisable pour 2020-2016) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD |
|
Segments couverts |
Par type de produit (instruments, consommables et accessoires), type de test (test d'imagerie, test sanguin, tests de moelle osseuse, test de biomarqueurs, immunophénotypage, tests génétiques et autres), type de cancer (myéloblastique (M0), myéloblastique (M1), myéloblastique (M2), promyélocytaire (M3), myélomonocytaire (M4), monocytaire (M5), érythroleucémie (M6) et mégacaryocytaire (M7)), groupe d'âge (moins de 21 ans, 21-29 ans, 30-65 ans et 65 ans et plus), sexe (homme et femme), utilisateur final (hôpital, laboratoires associés, laboratoires de diagnostic indépendants, centres d'imagerie diagnostique, instituts de recherche sur le cancer et autres), canal de distribution (appel d'offres direct et vente au détail). |
|
Pays couverts |
États-Unis, Canada et Mexique. |
|
Acteurs du marché couverts |
Myriad Genetics, Inc., Abbott, QIAGEN, Agilent Technologies, Inc., Exact Sciences Corporation, Hologic Inc., Illumina, Inc., BD, Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., FONAR Corp. et Thermo Fisher Scientific Inc. entre autres. |
Définition du marché
La forme la plus courante de cancer du sang, la leucémie myéloïde aiguë (LMA), est également l'une des formes rares de leucémie. Cette forme de cancer envahit le sang, qui se propage ensuite aux organes et systèmes corporels voisins. Les spécialistes doivent diagnostiquer manuellement les cellules cancéreuses et non cancéreuses en examinant les images cellulaires au microscope et en fournissant des étiquettes par annotation.
L’augmentation des dépenses de santé peut favoriser l’offre de R&D, qui stimule également la croissance des entreprises. La multiplication des laboratoires de recherche clinique et de diagnostic pour le dépistage préalable du cancer permet au marché de se développer.
Le nombre croissant de cas de cancer et la sensibilisation croissante à la leucémie ont entraîné une croissance accrue du marché.
Le marché nord-américain du diagnostic de la leucémie myéloïde aiguë connaît une croissance au cours de l'année de prévision en raison de l'augmentation du nombre d'acteurs sur le marché et de la disponibilité d'instruments d'imagerie et de consommables avancés. Parallèlement à cela, les fabricants sont engagés dans des activités de R&D pour lancer des produits sur le marché. L'augmentation de la R&D et des pré-tests stimule encore la croissance du marché. Cependant, les réglementations et normes strictes pour l'approbation et la commercialisation des produits de diagnostic du cancer et le diagnostic tardif devraient constituer un défi à la croissance du marché.
Dynamique du marché des diagnostics de la leucémie myéloïde aiguë en Amérique du Nord
Cette section traite de la compréhension des moteurs, des contraintes, des opportunités et des défis du marché. Tout cela est discuté en détail ci-dessous :
CONDUCTEURS
- Prévalence croissante du cancer de la leucémie
La leucémie peut toucher des personnes de tous âges. Elle peut être difficile à diagnostiquer car, malgré sa large gamme de signes et de symptômes, elle n'est pas spécifique et peut être liée à d'autres pathologies plus répandues. La LAM est l'un des quatre types de leucémie les plus courants chez l'adulte. Elle est moins fréquente. Elle est légèrement plus fréquente chez les hommes que chez les femmes. Cependant, le risque moyen de développer une LAM au cours de la vie chez les deux sexes est d'environ 0,5 % en moyenne.
La leucémie myéloïde aiguë (LMA) est le deuxième type de leucémie le plus fréquemment diagnostiqué chez les adultes et les enfants, mais la plupart des cas surviennent chez les adultes. Bien qu'elle puisse être diagnostiquée à tout âge, elle est rare avant 45 ans. L'âge moyen du diagnostic est de 68 ans. L'augmentation du taux d'obésité maternelle pourrait être en partie responsable de l'augmentation de la prévalence de la LMA.
En raison de divers facteurs de risque, l'incidence de la LAM est en hausse, devenant un problème socio-économique majeur. Cela devrait servir de moteur à la croissance du marché.
- Progrès technologiques dans le diagnostic de la leucémie
La forme la plus courante de cancer du sang, la LAM, est également l'une des formes rares de leucémie. Cette forme de cancer envahit le sang, qui se propage ensuite aux organes et systèmes corporels voisins. Les spécialistes doivent diagnostiquer manuellement les cellules cancéreuses et non cancéreuses en examinant les images cellulaires au microscope et en fournissant des étiquettes par annotation. Cependant, cet examen microscopique manuel prend du temps et peut donner un diagnostic erroné.
Le risque de prescription de médicaments erronés a été réduit grâce à des logiciels informatisés. La création d'un système de classification automatique et fiable est devenue essentielle pour mettre un terme aux effets dévastateurs de la leucémie. Les techniques de segmentation multiples ont constitué la base des algorithmes de classification de la leucémie existants.
Le développement de plusieurs nouvelles méthodes de diagnostic augmentera la croissance du marché à mesure que de nombreux produits nouveaux et avancés seront lancés. Par conséquent, on s'attend à ce qu'il crée une demande pour les produits de diagnostic du cancer leucémique aigu sur le marché.
RESTRICTIONS
- Réglementations et normes strictes pour l'approbation et la commercialisation des produits de diagnostic de la leucémie
Les réglementations strictes pour la commercialisation de tout produit sur le marché s'avèrent être un grand défi pour les fabricants de produits de diagnostic du cancer qui ont des réglementations et un organisme différent pour les procédures réglementaires.
Les fabricants doivent d'abord vérifier l'approbation du marquage CE avant de commercialiser leur produit sur le marché. Les politiques réglementaires strictes devraient freiner la croissance et le développement du marché.
- Diagnostic tardif et mauvais pronostic de la leucémie
Le cancer est la principale cause de mortalité dans le monde. Cependant, s'ils sont identifiés tôt et traités correctement, certains cancers peuvent être guéris. Le processus de diagnostic peut être retardé. Lorsque les patients négligent ou ne réagissent pas aux signes cancéreux potentiels, le diagnostic est retardé. La principale cause de présentation tardive est le manque de connaissance du public sur les signes précoces du cancer, en particulier si ces symptômes sont inhabituels.
Ces facteurs sont souvent à l'origine d'un diagnostic tardif, qui entraîne un mauvais pronostic et devrait donc freiner la croissance du marché.
OPPORTUNITÉ/DÉFIS
- Problèmes de coûts, de sécurité et de commodité accrus
La leucémie est un cancer mortel et son diagnostic pose également des problèmes de sécurité. Il n’est pas rentable. Le cancer est l’un des troubles médicaux les plus coûteux à traiter. Les patients atteints de cancer peuvent être hospitalisés et recevoir diverses thérapies, telles que la chirurgie, la radiothérapie et la thérapie systémique. Les primes d’assurance maladie pour les patients atteints de cancer sont désormais plus chères que par le passé. De plus, les coûts de leur quote-part, de leur franchise et de leur coassurance augmentent.
Désormais, le processus actuel de diagnostic de la leucémie pose des problèmes de sécurité, de coût et de commodité, qui devraient constituer un défi à la croissance du marché.
Développements récents
- En novembre 2022, Canon Inc. a acquis Redlen Technologies Inc. (Redlen), l'une des principales entreprises mondiales dans la création de nouvelles technologies liées au développement et à la fabrication de modules de détection à semi-conducteurs. Canon Medical Systems Corporation (Canon Medical), une société du groupe Canon Inc., a développé le premier système de tomodensitométrie à comptage de photons (PCCT) de fabrication nationale intégrant les technologies avancées de Redlen. Ce système a été installé au Centre de recherche et d'essais cliniques en oncologie exploratoire du National Cancer Center (NCC) au Japon, où il est actuellement utilisé pour mener des recherches explorant les applications cliniques du PCCT.
- En octobre 2022, le président-directeur général de Sysmex Corporation, Hisashi Ietsugu, a annoncé l'approbation d'une demande de modification partielle de l'autorisation de fabrication et de commercialisation au Japon de son réactif d'amplification génique LYNOAMP CK19 commercialisé comme réactif de test de métastase des ganglions lymphatiques pour le cancer du sein, le cancer colorectal, le cancer gastrique et le cancer du poumon non à petites cellules, étendant son amplification au cancer du col de l'utérus et au cancer de l'endomètre.
Portée du marché nord-américain du diagnostic de la leucémie myéloïde aiguë
Le marché nord-américain du diagnostic de la leucémie myéloïde aiguë est segmenté en sept segments notables en fonction du type de produit, du type de test, du type de cancer, de la tranche d'âge, du sexe, de l'utilisateur final et du canal de distribution. La croissance de ces segments vous aidera à analyser les principaux segments de croissance des industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour prendre des décisions stratégiques afin d'identifier les principales applications du marché.
Type de produit
- Instruments
- Consommables et accessoires
En fonction du type de produit, le marché est segmenté en instruments et consommables et accessoires.
Type de test
- Analyse de sang
- Test d'imagerie
- Test de moelle osseuse
- Test génétique
- Test de biomarqueurs
- Immunophénotypage
- Autres
En fonction du type de test, le marché est segmenté en test sanguin, test d'imagerie, test de moelle osseuse, test génétique, test de biomarqueurs, immunophénotypage et autres.
Type de cancer
- Myéloblastique (M0)
- Myéloblastique (M1)
- Myéloblastique (M2)
- Promyélocytaire (M3)
- Myélomonocytaire (M4)
- Monocytaire (M5)
- Érythroleucémie (M6)
- Mégacaryocytaire (M7)
En fonction du type de cancer, le marché est segmenté en myéloblastique (M0), myéloblastique (M1), myéloblastique (M2), promyélocytaire (M3), myélomonocytaire (M4), monocytaire (M5), érythroleucémique (M6) et mégacaryocytaire (M7).
Groupe d'âge
- Moins de 21 ans
- 21-29
- 30-65
- 65 ans et plus
En fonction de la tranche d’âge, le marché est segmenté en moins de 21 ans, 21-29 ans, 30-65 ans et 65 ans et plus.
Genre
- Mâle
- Femelle
En fonction du sexe, le marché est segmenté en hommes et femmes.
Utilisateur final
- Hôpital
- Laboratoires associés
- Laboratoires de diagnostic indépendants
- Centres d'imagerie diagnostique
- Instituts de recherche sur le cancer
- Autres
En fonction de l’utilisateur final, le marché est segmenté en hôpitaux, laboratoires associés, laboratoires de diagnostic indépendants, centres d’imagerie diagnostique, instituts de recherche sur le cancer et autres.
Canal de distribution
- Appel d'offres direct
- Ventes au détail
En fonction du canal de distribution, le marché est segmenté en ventes directes et ventes au détail.
Analyse/perspectives régionales du marché du diagnostic de la leucémie myéloïde aiguë en Amérique du Nord
Le marché nord-américain du diagnostic de la leucémie myéloïde aiguë est analysé et des informations et tendances sur la taille du marché sont fournies par pays en fonction du type de produit, du type de test, du type de cancer, de la tranche d'âge, du sexe, de l'utilisateur final et du canal de distribution.
Les pays couverts dans ce rapport de marché sont les États-Unis, le Canada et le Mexique, comme indiqué ci-dessus.
Les États-Unis dominent le marché nord-américain du diagnostic de la leucémie myéloïde aiguë en termes de part de marché et de chiffre d'affaires et continueront de renforcer leur domination au cours de la période de prévision. Cela est dû aux avancées technologiques croissantes en matière de diagnostic dans la région, à la croissance des investissements en R&D et au lancement de nouveaux produits qui stimulent la croissance du marché.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données, tels que les ventes de produits neufs et de remplacement, la démographie des pays, l'épidémiologie des maladies et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario du marché pour les différents pays. En outre, la présence et la disponibilité des marques nord-américaines et les défis auxquels elles sont confrontées en raison de la concurrence des marques locales et nationales et l'impact des canaux de vente sont pris en compte tout en fournissant une analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché du diagnostic de la leucémie myéloïde aiguë en Amérique du Nord
Le paysage concurrentiel du marché nord-américain des diagnostics de leucémie myéloïde aiguë fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements en R&D, les nouvelles initiatives du marché, la présence en Amérique du Nord, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises par rapport au marché.
Certains des principaux acteurs opérant sur le marché du diagnostic de la leucémie myéloïde aiguë en Amérique du Nord sont Myriad Genetics, Inc., Abbott, QIAGEN, Agilent Technologies, Inc., Exact Sciences Corporation, Hologic Inc., Illumina, Inc., BD, Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., FONAR Corp. et Thermo Fisher Scientific Inc. entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
5 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, REGULATIONS
5.1 REGULATORY SCENARIO IN THE U.S.
5.2 REGULATORY SCENARIO IN AUSTRALIA
5.3 REGULATORY SCENARIO IN JAPAN
5.4 REGULATORY SCENARIO IN CHINA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING PREVALENCE OF LEUKEMIA CANCER
6.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN LEUKEMIA DIAGNOSTICS
6.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
6.1.4 INCREASE IN AWARENESS REGARDING LEUKEMIA CANCER
6.2 RESTRAINTS
6.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF LEUKEMIA DIAGNOSTIC PRODUCTS
6.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF LEUKEMIA
6.3 OPPORTUNITIES
6.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR LEUKEMIA
6.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
6.3.3 GOVERNMENT INITIATIVES TOWARD CANCER DIAGNOSTICS
6.4 CHALLENGES
6.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
6.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 INSTRUMENTS
7.2.1 BIOPSY INSTRUMENTS
7.2.1.1 BONE MARROW BIOPSY
7.2.1.2 NEEDLE BIOPSY
7.2.1.3 SURGICAL BIOPSY
7.2.1.4 OTHERS
7.2.2 PATHOLOGY-BASED INSTRUMENTS
7.2.2.1 PCR INSTRUMENTS
7.2.2.2 SLIDE STAINING SYSTEMS
7.2.2.3 TISSUE PROCESSING SYSTEMS
7.2.2.4 CELL PROCESSORS
7.2.2.5 OTHER PATHOLOGY-BASED INSTRUMENTS
7.2.3 IMAGING INSTRUMENTS
7.2.3.1 ULTRASOUND SYSTEMS
7.2.3.2 CT SYSTEMS
7.2.3.3 MRI SYSTEMS
7.2.3.4 OTHERS
7.2.4 OTHERS
7.3 CONSUMABLES & ACCESSORIES
7.3.1 KITS
7.3.1.1 PCR KITS
7.3.1.2 DNA POLYMERASE KITS
7.3.1.3 NUCLEIC ACID ISOLATION KITS
7.3.1.4 OTHERS
7.3.2 REAGENTS
7.3.2.1 ASSAYS
7.3.2.2 BUFFERS
7.3.2.3 PRIMERS
7.3.2.4 OTHERS
7.3.3 PROBES
7.3.4 OTHER CONSUMABLES
8 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 BLOOD TEST
8.2.1 COMPLETE BLOOD COUNT (CBC)
8.2.2 BLOOD CHEMISTRY TESTS
8.2.3 OTHERS
8.3 IMAGING TEST
8.3.1 COMPUTED TOMOGRAPHY (CT) SCAN
8.3.2 MRI
8.3.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
8.3.4 OTHERS
8.4 BONE MARROW TESTS
8.4.1 BONE MARROW ASPIRATE
8.4.2 BONE MARROW BIOPSY
8.4.3 OTHERS
8.5 GENETIC TESTS
8.5.1 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
8.5.2 KARYOTYPING
8.5.3 OTHERS
8.6 BIOMARKER TEST
8.6.1 GENETIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.6.2 EPIGENETIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.6.3 PROTEOMIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.7 IMMUNOPHENOTYPING
8.7.1 FLOW CYTOMETRY
8.7.2 IMMUNOHISTOCHEMISTRY
8.7.3 OTHERS
8.8 OTHERS
9 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE
9.1 OVERVIEW
9.2 MYELOBLASTIC (M0)
9.3 MYELOBLASTIC (M1)
9.4 MYELOBLASTIC (M2)
9.5 PROMYELOCYTIC (M3)
9.6 MYELOMONOCYTIC (M4)
9.7 MONOCYTIC (M5)
9.8 ERYTHROLEUKEMIA (M6)
9.9 MEGAKARYOCYTIC (M7)
10 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP
10.1 OVERVIEW
10.2 65 AND ABOVE
10.3 30-65
10.4 BELOW 21
10.5 21-29
11 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER
11.1 OVERVIEW
11.2 MALE
11.3 FEMALE
12 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 ASSOCIATED LABS
12.4 INDEPENDENT DIAGNOSTIC LABORATORIES
12.5 DIAGNOSTIC IMAGING CENTERS
12.6 CANCER RESEARCH INSTITUTES
12.7 OTHERS
13 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
14 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
15 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 CANON MEDICAL SYSTEMS CORPORATION.
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 SYSMEX CORPORATION
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 EPIGENOMICS AG.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 MYRIAD GENETICS, INC..
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 F. HOFFMANN- LA ROCHE LTD
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 ABBOTT
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 AGILENT TECHNOLOGIES, INC.
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 BD
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENT
17.9 BIOMERIEUX
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENT
17.1 BIO-RAD LABORATORIES, INC.
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 DIASORIN S.P.A.
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 EXACT SCIENCES CORPORATION
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 FONAR CORP.
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 HOLOGIC INC.
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENT
17.15 ILLUMINA, INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 KONINKLIJKE PHILIPS N.V.
17.16.1 COMPANY SNAPSHOT
17.16.2 REVENUE ANALYSIS
17.16.3 PRODUCT PORTFOLIO
17.16.4 RECENT DEVELOPMENT
17.17 MEDONICA CO. LTD
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENT
17.18 MERCK KGAA
17.18.1 COMPANY SNAPSHOT
17.18.2 REVENUE ANALYSIS
17.18.3 PRODUCT PORTFOLIO
17.18.4 RECENT DEVELOPMENTS
17.19 MINFOUND MEDICAL SYSTEMS CO., LTD
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 PLEXBIO
17.20.1 COMPANY SNAPSHOT
17.20.2 PRODUCT PORTFOLIO
17.20.3 RECENT DEVELOPMENTS
17.21 QIAGEN
17.21.1 COMPANY SNAPSHOT
17.21.2 REVENUE ANALYSIS
17.21.3 PRODUCT PORTFOLIO
17.21.4 RECENT DEVELOPMENTS
17.22 QUEST DIAGNOSTICS INCORPORATED
17.22.1 COMPANY SNAPSHOT
17.22.2 REVENUE ANALYSIS
17.22.3 PRODUCT PORTFOLIO
17.22.4 RECENT DEVELOPMENTS
17.23 SIEMENS HEALTHCARE GMBH
17.23.1 COMPANY SNAPSHOT
17.23.2 REVENUE ANALYSIS
17.23.3 PRODUCT PORTFOLIO
17.23.4 RECENT DEVELOPMENT
17.24 SONIC HEALTHCARE
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENT
17.25 STERNMED GMBH
17.25.1 COMPANY SNAPSHOT
17.25.2 PRODUCT PORTFOLIO
17.25.3 RECENT DEVELOPMENTS
17.26 THERMO FISHER SCIENTIFIC INC.
17.26.1 COMPANY SNAPSHOT
17.26.2 REVENUE ANALYSIS
17.26.3 PRODUCT PORTFOLIO
17.26.4 RECENT DEVELOPMENT
17.27 TIME MEDICAL HOLDING
17.27.1 COMPANY SNAPSHOT
17.27.2 PRODUCT PORTFOLIO
17.27.3 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
Liste des tableaux
TABLE 1 APPROVED DIAGNOSTICS OF LEUKEMIA
TABLE 2 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA CONSUMABLES AND ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA GENETIC TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA GENETIC TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA OTHERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA MYELOBLASTIC (M0) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA MYELOBLASTIC (M1) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA MYELOBLASTIC (M2) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA PROMYELOCYTIC (M3) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA MYELOMONOCYTIC (M4) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA MONOCYTIC (M5) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA ERYTHROLEUKEMIA (M6) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA MEGAKARYOCYTIC (M7) ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA 65 AND ABOVE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA 30-65 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA BELOW 21 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA 21-29 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA MALE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA FEMALE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA HOSPITALS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA ASSOCIATED LABS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA INDEPENDENT DIAGNOSTIC LABORATORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA DIAGNOSTIC IMAGING CENTERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA CANCER RESEARCH INSTITUTES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA OTHERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA DIRECT TENDER IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA RETAIL SALES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY COUNTRY
TABLE 54 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 74 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 U.S. INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 U.S. PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 77 U.S. IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 78 U.S. BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 U.S. CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.S. KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.S. REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.S. IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.S. BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.S. BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 86 U.S. BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 U.S. IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 88 U.S. GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 90 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 91 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 92 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 93 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 94 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 95 CANADA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 CANADA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 CANADA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 CANADA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 CANADA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 CANADA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 CANADA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 CANADA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 CANADA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 CANADA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 CANADA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 CANADA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 CANADA GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 110 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 111 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 112 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 MEXICO INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 MEXICO PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 MEXICO IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 MEXICO BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 MEXICO CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 MEXICO KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 121 MEXICO REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 122 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 123 MEXICO IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 124 MEXICO BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 125 MEXICO BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 126 MEXICO BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 127 MEXICO IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 MEXICO GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 130 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 131 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 132 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 133 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 2 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DROC ANALYSIS
FIGURE 4 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF LEUKEMIA CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INSTRUMENTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET
FIGURE 14 NUMBER OF PREVALENCE OF LEUKEMIA INCIDENCE WORLDWIDE (BOTH SEXES)
FIGURE 15 FIVE YEARS PREVALENCE LEUKEMIA INCIDENCE WORLDWIDE (BOTH SEXES)
FIGURE 16 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 17 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 18 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 19 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 21 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 22 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 23 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 25 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 26 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 27 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, 2022
FIGURE 29 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 30 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, CAGR (2023-2030)
FIGURE 31 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 32 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, 2022
FIGURE 33 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, 2023-2030 (USD MILLION)
FIGURE 34 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, CAGR (2023-2030)
FIGURE 35 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 36 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 37 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 38 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 39 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 41 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 42 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 43 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 45 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 46 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 47 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 48 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 49 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.