Middle East Molecular Point Of Care Testing Using Naat Market
Taille du marché en milliards USD
TCAC :
% 
 USD
274.00 Million
USD
484.16 Million
2024
2032
USD
274.00 Million
USD
484.16 Million
2024
2032
| 2025 –2032 | |
| USD 274.00 Million | |
| USD 484.16 Million | |
|
|
|
|
Segmentation du marché des tests moléculaires au point d'intervention (par TAAN) au Moyen-Orient, par produit (instruments, consommables et réactifs), indication (tests d'infections respiratoires, tests d'infections sexuellement transmissibles (IST), tests d'infections gastro-intestinales et autres), utilisateur final (laboratoires, hôpitaux, cliniques, centres ambulatoires, soins à domicile, résidences services, etc.), mode de test (tests sur ordonnance et tests en vente libre), canal de distribution (pharmacies hospitalières, pharmacies de détail et pharmacies en ligne) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des tests moléculaires au point de service (par TAAN) au Moyen-Orient
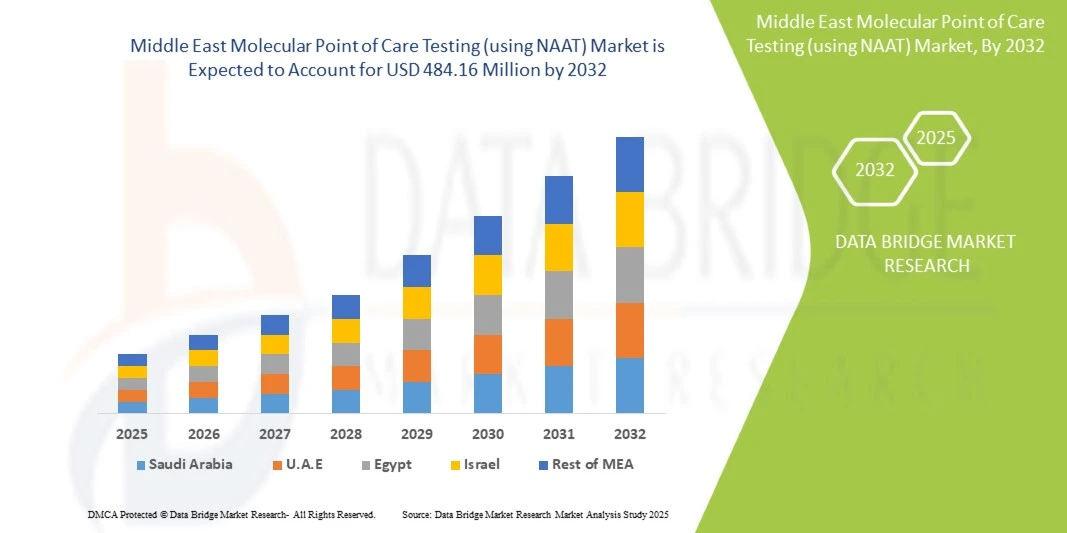
- La taille du marché des tests moléculaires au point de service (utilisant NAAT) au Moyen-Orient était évaluée à 274,00 millions USD en 2024 et devrait atteindre 484,16 millions USD d'ici 2032 , à un TCAC de 7,4 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par la prévalence croissante des maladies infectieuses, les avancées technologiques dans le domaine du diagnostic moléculaire et l’adoption croissante des tests au point de service dans les hôpitaux, les cliniques et les laboratoires de diagnostic.
- Par ailleurs, la croissance des dépenses de santé, les initiatives gouvernementales visant à améliorer l'accessibilité au diagnostic et la demande de solutions de dépistage rapides, précises et décentralisées font du test NAAT au point de service un outil de diagnostic privilégié dans la région. Ces facteurs convergents accélèrent l'adoption de solutions moléculaires au point de service, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché des tests moléculaires au point de service (tests NAAT) au Moyen-Orient
- Les tests moléculaires au point de service utilisant le TAAN, offrant une détection rapide et précise des maladies infectieuses sur ou à proximité du lieu de soins aux patients, sont de plus en plus essentiels dans les systèmes de santé modernes des hôpitaux, des cliniques et des laboratoires de diagnostic en raison de leur grande sensibilité, de leur délai d'exécution rapide et de leur facilité d'intégration dans les flux de travail cliniques.
- La demande croissante de tests de dépistage au point de service basés sur le TAAN est principalement alimentée par la prévalence croissante des maladies infectieuses, la sensibilisation croissante aux diagnostics précoces et une préférence pour les solutions de test décentralisées et rapides par rapport aux tests de laboratoire traditionnels.
- L'Arabie saoudite a dominé le marché des tests de dépistage au point de service basés sur le test NAAT au Moyen-Orient avec la plus grande part de revenus de 38,5 % en 2024, caractérisée par une infrastructure de soins de santé avancée, des dépenses de santé élevées et une forte présence d'acteurs clés de l'industrie, avec une croissance substantielle tirée par les innovations des sociétés de diagnostic établies et des startups locales émergentes.
- Les Émirats arabes unis devraient être le pays connaissant la croissance la plus rapide sur le marché au cours de la période de prévision, en raison de l'augmentation des investissements dans les soins de santé, des initiatives gouvernementales visant à améliorer l'accès au diagnostic et de l'adoption croissante de technologies de diagnostic modernes.
- Le segment des tests d'infections respiratoires a dominé le marché avec une part de 50,5 % en 2024, grâce à la forte prévalence des infections virales et bactériennes et à la fiabilité clinique établie de la technologie NAAT pour une détection rapide et précise.
Portée du rapport et segmentation du marché des tests moléculaires au point de service (à l'aide du TAAN) au Moyen-Orient
|
Attributs |
Analyses moléculaires au point de service (tests NAAT) : principales perspectives du marché au Moyen-Orient |
|
Segments couverts |
|
|
Pays couverts |
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des tests moléculaires au point de service (tests NAAT) au Moyen-Orient
Tests rapides et décentralisés pour les maladies infectieuses
- Une tendance importante et croissante sur le marché des tests de dépistage au point de service basés sur le TAAN au Moyen-Orient est l'adoption croissante de solutions de diagnostic rapides et décentralisées qui peuvent fournir des résultats très précis à proximité du site de soins du patient, réduisant ainsi la dépendance aux laboratoires centraux.
- Par exemple, la plateforme Cepheid GeneXpert est largement déployée dans les hôpitaux et cliniques d'Arabie saoudite et des Émirats arabes unis, fournissant des résultats de dépistage de proximité pour la tuberculose, la COVID-19 et les infections sexuellement transmissibles. De même, les dispositifs Abbott ID NOW sont déployés dans les établissements de santé communautaires pour une détection rapide de la grippe et de la COVID-19.
- L'intégration aux plateformes de santé numériques et aux systèmes d'information de laboratoire permet des rapports en temps réel, des alertes automatisées et une meilleure prise en charge des patients. Par exemple, aux Émirats arabes unis, les appareils NAAT POCT connectés aux systèmes de dossiers médicaux électroniques des hôpitaux permettent aux cliniciens de recevoir des notifications instantanées en cas de cas positifs, accélérant ainsi les interventions.
- La tendance vers des dispositifs de test NAAT portables, conviviaux et multiplexés favorise une plus grande accessibilité, permettant aux cliniques et même aux centres de test distants d'effectuer des diagnostics rapides pour de multiples maladies infectieuses.
- Cette évolution vers des tests rapides, précis et décentralisés redéfinit les attentes diagnostiques dans la région. Des entreprises comme bioMérieux et QIAGEN développent des dispositifs TAAN compacts et utilisables au point de service, dotés de fonctions intégrées de reporting et de télésurveillance, améliorant ainsi la rapidité et la fiabilité de la détection des maladies infectieuses.
- La demande de solutions POCT moléculaires rapides, décentralisées et intégrées numériquement augmente dans les hôpitaux, les cliniques et les centres de tests communautaires, car les prestataires de soins de santé accordent de plus en plus la priorité à un diagnostic plus rapide et à une meilleure gestion des patients.
Dynamique du marché des tests moléculaires au point de service (tests NAAT) au Moyen-Orient
Conducteur
Prévalence croissante des maladies infectieuses et investissements dans les soins de santé
- La charge croissante des maladies infectieuses telles que la COVID-19, la grippe, la tuberculose et les infections sexuellement transmissibles dans les pays du Moyen-Orient, associée à des investissements croissants dans les infrastructures de santé, est un facteur important de l'adoption croissante des tests de dépistage au point de service basés sur le test NAAT.
- Par exemple, en 2024, le ministère saoudien de la Santé a étendu ses programmes de dépistage rapide aux hôpitaux et aux centres de soins primaires, en déployant des dispositifs TAAN pour la détection précoce de la COVID-19 et des infections respiratoires. De telles initiatives des gouvernements et des organismes de santé devraient stimuler la croissance du marché durant la période de prévision.
- Alors que les cliniciens et les autorités de santé publique accordent la priorité au diagnostic rapide et à la gestion efficace des patients, les solutions NAAT POCT offrent un délai d'exécution rapide, une sensibilité élevée et une détection précise, offrant une alternative convaincante aux tests de laboratoire centralisés traditionnels.
- En outre, l’adoption croissante des dossiers médicaux électroniques, de la télémédecine et des plateformes de santé numériques crée un environnement favorable aux solutions POCT intégrées, permettant une gestion transparente des données et des rapports en temps réel.
- Le besoin de solutions de diagnostic rapides, précises et décentralisées, associé au soutien du gouvernement et aux investissements dans les infrastructures de santé, propulse l'adoption de tests de dépistage au point de service basés sur le NAAT dans les secteurs de la santé publics et privés.
Retenue/Défi
Coûts élevés et main-d'œuvre qualifiée limitée
- Le coût relativement élevé des dispositifs, consommables et réactifs de dépistage des tumeurs non invasives (TAAN) reste un obstacle majeur à une pénétration plus large du marché, notamment dans les petites cliniques et les hôpitaux aux budgets limités. Si les prix baissent progressivement, l'investissement initial pour les dispositifs de tests moléculaires avancés peut freiner leur adoption.
- Par exemple, des hôpitaux plus petits en Égypte et au Qatar ont signalé des limitations budgétaires lors de l’achat de plateformes NAAT avancées auprès d’entreprises telles que Cepheid et Abbott, ce qui limite le déploiement à grande échelle.
- Un autre défi majeur réside dans la pénurie de personnel de laboratoire et de clinique qualifié, capable d'utiliser les dispositifs TAAN avec précision et d'interpréter les résultats. Une mauvaise gestion ou une manipulation inappropriée peut affecter la fiabilité des tests et les résultats pour les patients.
- Relever ces défis grâce à des programmes de formation, des subventions gouvernementales et des plateformes NAAT rentables sera essentiel pour assurer une croissance durable du marché. Des entreprises comme bioMérieux et QIAGEN privilégient des interfaces conviviales, la formation à distance et un accompagnement pour favoriser l'adoption dans les contextes à ressources limitées.
- Surmonter les coûts élevés et les limitations de main-d’œuvre grâce à des solutions NAAT innovantes, faciles à utiliser et abordables sera essentiel pour élargir l’accès et stimuler la croissance du marché à long terme au Moyen-Orient.
Portée du marché des tests moléculaires au point de service (à l'aide du TAAN) au Moyen-Orient
Le marché est segmenté sur la base du produit, de l’indication, de l’utilisateur final, du mode de test et du canal de distribution.
- Par produit
En fonction du produit, le marché est segmenté en instruments et en consommables et réactifs. En 2024, les instruments ont dominé le marché, générant la plus grande part de chiffre d'affaires en raison de leur rôle essentiel dans les tests TAAN et de leur coût initial élevé. Les hôpitaux, les cliniques et les laboratoires de référence privilégient les investissements dans des instruments de pointe pour garantir des résultats précis et fiables. Ces instruments prennent en charge plusieurs analyses et tests multiplex, permettant une gestion efficace des diagnostics respiratoires, gastro-intestinaux et des IST. Les gouvernements et les prestataires de soins de santé d'Arabie saoudite et des Émirats arabes unis déploient ces dispositifs dans le cadre de programmes de santé publique afin d'améliorer les délais de diagnostic. Leur durabilité, leur automatisation et leur intégration aux plateformes de santé numérique les rendent indispensables aux flux de travail hospitaliers. De grandes entreprises telles que Cepheid, Abbott et bioMérieux proposent des instruments compacts et automatisés conçus pour les tests au plus près du patient, ce qui favorise leur adoption.
Les consommables et réactifs devraient connaître la croissance la plus rapide entre 2025 et 2033, portée par la demande récurrente de cartouches de test, de kits d'extraction et de réactifs à chaque test. La prévalence croissante des maladies infectieuses et le développement des centres de dépistage communautaires stimulent la demande. Les réactifs multiplex, capables de tester simultanément plusieurs agents pathogènes, améliorent la rentabilité et attirent les professionnels de santé. Les cliniques émergentes et les petits hôpitaux privilégient ces consommables pour des tests flexibles et rapides, sans avoir à investir dans de multiples instruments. L'intensification des campagnes de dépistage menées par les gouvernements et l'intégration de la télésanté favorisent encore leur adoption. La croissance de ces consommables assure des revenus stables aux entreprises de diagnostic.
- Par indication
Sur la base des indications, le marché est segmenté en tests d'infections respiratoires, d'infections sexuellement transmissibles (IST), d'infections gastro-intestinales, etc. Les tests d'infections respiratoires ont dominé le marché en 2024 en raison de la forte prévalence de maladies telles que la COVID-19, la grippe et le VRS au Moyen-Orient. Les hôpitaux et les cliniques s'appuient sur les tests NAAT rapides pour une prise en charge rapide des patients et le contrôle des infections. Les gouvernements d'Arabie saoudite et des Émirats arabes unis ont lancé des initiatives de dépistage de masse, stimulant encore la demande. La haute sensibilité et la rapidité d'exécution des tests NAAT les rendent préférables aux méthodes antigéniques ou basées sur la culture. La domination de ce segment est renforcée par les épidémies saisonnières et les mesures de préparation aux pandémies. Les prestataires de soins de santé accordent une priorité aux tests respiratoires, car ils ont un impact direct sur les décisions thérapeutiques et l'efficacité des flux de travail hospitaliers.
Le dépistage des infections sexuellement transmissibles (IST) devrait connaître sa croissance la plus rapide entre 2025 et 2033, grâce à une sensibilisation accrue et aux programmes de dépistage soutenus par les gouvernements. Les tests d'infection par TAAN offrent une sensibilité et une spécificité élevées pour la chlamydia, la gonorrhée et d'autres IST, ce qui les rend idéaux pour le diagnostic à proximité du patient. Leur adoption croissante dans les cliniques et les centres ambulatoires pour des tests discrets et rapides stimule leur expansion. La prévalence croissante des IST dans les populations urbaines et la demande croissante de solutions de dépistage privées soutiennent la croissance rapide de ce segment. Les panels TAAN multiplex pour la détection des IST améliorent encore l'efficacité et la commodité. Le développement de la télésanté et du prélèvement d'échantillons à domicile favorise également l'adoption du TAAN pour les IST.
- Par utilisateur final
En fonction de l'utilisateur final, le marché est segmenté en laboratoires, hôpitaux, cliniques, centres ambulatoires, soins à domicile, résidences services, etc. En 2024, les hôpitaux ont dominé le marché en raison de l'important volume de patients et du besoin de diagnostics rapides et fiables. Ils bénéficient de leurs propres instruments TAAN qui réduisent les délais de diagnostic et améliorent la prévention des infections. Leur déploiement aux urgences, en hospitalisation et en consultation externe garantit une demande continue. Les hôpitaux publics et privés d'Arabie saoudite et des Émirats arabes unis adoptent le TAAN POCT pour leurs programmes nationaux de dépistage. La combinaison de la capacité d'accueil, des besoins cliniques et des investissements en infrastructures fait des hôpitaux leur principal contributeur au chiffre d'affaires. Ils s'appuient également sur des systèmes de reporting intégrés pour un suivi en temps réel des tendances des maladies infectieuses.
Les cliniques devraient être le segment d'utilisateurs finaux connaissant la croissance la plus rapide entre 2025 et 2033, stimulé par la décentralisation des tests et la demande des patients pour des diagnostics à proximité du patient. Les cliniques peuvent fournir des résultats le jour même, réduisant ainsi les orientations vers des laboratoires centraux et améliorant la satisfaction des patients. La croissance des centres de soins primaires et des cliniques communautaires aux Émirats arabes unis, en Égypte et au Qatar stimule l'adoption de ces dispositifs. Les cliniques privilégient les appareils TAAN compacts et conviviaux, nécessitant une formation minimale du personnel. La tendance aux bilans de santé préventifs et au dépistage systématique des maladies infectieuses accélère encore la croissance. L'intégration croissante aux plateformes de télémédecine améliore la praticité et l'évolutivité des tests de dépistage rapide en clinique.
- Par mode de test
Selon le mode de test, le marché est segmenté en tests sur ordonnance et en tests en vente libre. En 2024, les tests sur ordonnance ont dominé le marché en raison des exigences de supervision médicale pour les tests TAAN et de la conformité réglementaire. Les hôpitaux et les cliniques proposent des tests sur ordonnance afin de garantir une manipulation et une interprétation précises. En Arabie saoudite, aux Émirats arabes unis et en Égypte, les réglementations imposent une supervision professionnelle pour les diagnostics moléculaires. Le coût élevé et la formation spécialisée renforcent la prédominance des tests sur ordonnance. Cette approche garantit le contrôle qualité et réduit le risque d'erreur de diagnostic. La plupart des dépistages et des déploiements hospitaliers à grande échelle reposent sur des solutions TAAN sur ordonnance.
Les tests en vente libre devraient connaître la croissance la plus rapide entre 2025 et 2033, en raison de la demande croissante de diagnostics à domicile et de l'intégration de la télésanté. Les patients peuvent effectuer des tests TAAN pour la COVID-19, la grippe et d'autres infections à domicile et partager les résultats avec les professionnels de santé à distance. Cette approche améliore l'accessibilité et la commodité, notamment dans les zones urbaines dynamiques. Les plateformes numériques permettent l'orientation et la communication d'informations à distance, ce qui favorise l'adoption de ces tests. La sensibilisation croissante aux soins préventifs et à l'autodiagnostic stimule l'expansion de ce segment. Les tests TAAN en vente libre offrent une flexibilité aux patients tout en allégeant la pression sur les hôpitaux et les cliniques.
- Par canal de distribution
En fonction du canal de distribution, le marché est segmenté en pharmacies hospitalières, pharmacies de détail et pharmacies en ligne. Les pharmacies hospitalières ont dominé le marché en 2024 grâce à la centralisation des achats pour les hôpitaux et les cliniques. Elles gèrent efficacement l'approvisionnement en instruments, kits de test et consommables pour plusieurs services. L'approvisionnement en gros garantit la rentabilité et un approvisionnement régulier en réactifs. Les hôpitaux s'appuient sur une logistique gérée par les pharmacies pour assurer la continuité des services de test. L'intégration avec les systèmes informatiques hospitaliers permet la gestion des stocks et le réapprovisionnement en temps réel. Les pharmacies hospitalières restent le principal canal de revenus des entreprises de tests NAAT POCT dans la région.
La pharmacie en ligne devrait connaître la croissance la plus rapide entre 2025 et 2033, grâce à l'adoption croissante de la santé numérique au Moyen-Orient. Les patients peuvent commander des kits TAAN pour des tests à domicile avec livraison, bénéficiant ainsi d'un accès pratique et discret. Les plateformes de télésanté permettent l'orientation et la soumission des résultats à distance. La croissance du e-commerce et des applications mobiles de santé accélère l'adoption de ce canal. Les tests à domicile et le suivi à distance renforcent l'engagement des patients. Les pharmacies en ligne offrent un accès aux populations urbaines et isolées, ce qui en fait un segment de distribution en pleine expansion.
Analyse régionale du marché des tests moléculaires au point de service (par TAAN) au Moyen-Orient
- L'Arabie saoudite a dominé le marché des tests de dépistage au point de service basés sur le test NAAT au Moyen-Orient avec la plus grande part de revenus de 38,5 % en 2024, caractérisée par une infrastructure de soins de santé avancée, des dépenses de santé élevées et une forte présence d'acteurs clés de l'industrie, avec une croissance substantielle tirée par les innovations des sociétés de diagnostic établies et des startups locales émergentes.
- Les professionnels de santé et les autorités de santé publique d'Arabie saoudite apprécient grandement la précision, la rapidité et la fiabilité du test d'amplification des acides nucléiques (TAAN) au point de vente pour la prise en charge des infections respiratoires, des IST et autres maladies infectieuses. Un diagnostic rapide permet un traitement rapide, la maîtrise des épidémies et l'amélioration des résultats pour les patients.
- Cette adoption généralisée est également soutenue par des dépenses de santé élevées, un personnel médical à la pointe de la technologie et des partenariats solides avec des entreprises mondiales de diagnostic de premier plan. Les hôpitaux, les cliniques et les laboratoires intègrent de plus en plus les appareils NAAT POCT à leurs systèmes d'information hospitaliers, permettant ainsi un reporting en temps réel et une gestion efficace des patients.
Aperçu du marché des tests moléculaires au point de service (tests NAAT) en Arabie saoudite
Le marché saoudien des tests moléculaires au point de service (par TAAN) a représenté la plus grande part de chiffre d'affaires en 2024, avec 38,5 %, grâce à la forte prévalence des maladies infectieuses et à l'infrastructure de santé avancée. Les hôpitaux, les cliniques et les laboratoires adoptent de plus en plus les diagnostics basés sur le TAAN pour une détection rapide et précise des infections respiratoires, des IST et des infections gastro-intestinales. Les programmes nationaux de dépistage du gouvernement et les initiatives de préparation aux pandémies stimulent encore davantage cette adoption. L'augmentation des investissements dans les technologies de la santé et l'intégration des dispositifs TAAN aux plateformes de santé numériques, notamment les systèmes d'information hospitaliers et la télémédecine, élargissent la portée du marché. L'accent croissant mis sur les soins de santé préventifs et les mesures de contrôle des infections continue de stimuler la croissance du marché.
Analyse du marché des tests moléculaires au point de service (TAAN) aux Émirats arabes unis
Le marché des tests moléculaires délocalisés aux Émirats arabes unis est en passe de devenir le pays connaissant la croissance la plus rapide de la région, grâce à l'augmentation des investissements dans la santé et au développement des réseaux hospitaliers. L'adoption des tests TAAN est soutenue par les programmes gouvernementaux de surveillance des maladies infectieuses et d'intégration de la télésanté. Les cliniques et les hôpitaux adoptent des tests rapides et à proximité du patient pour un diagnostic rapide et une meilleure prise en charge des patients. Le système de santé technologiquement avancé des Émirats arabes unis, associé à une forte sensibilisation des patients aux diagnostics précoces, stimule la croissance du marché. La demande croissante de tests TAAN multiplex et de solutions de soins à domicile contribue également à leur adoption.
Analyse du marché égyptien des tests moléculaires au point de service (à l'aide du TAAN)
Le marché égyptien des tests NAAT (tests d'amplification des acides nucléiques) sur le lieu de travail (POCT) a représenté une part significative en 2024, grâce à l'amélioration des infrastructures de santé et à la sensibilisation croissante aux diagnostics moléculaires rapides. Les hôpitaux et les centres de soins primaires adoptent de plus en plus les tests NAAT pour les infections respiratoires et les IST. Les initiatives gouvernementales soutenant les tests décentralisés et les programmes de dépistage communautaire renforcent la croissance du marché. Le développement des plateformes de télémédecine et des systèmes de reporting numérique améliore l'accessibilité et la commodité. La prévalence croissante des maladies infectieuses et la demande croissante des patients pour un diagnostic plus rapide soutiennent le marché. L'accessibilité financière et les partenariats locaux avec des entreprises de diagnostic stimulent également l'adoption de ces tests.
Analyse du marché des tests moléculaires au point de service (tests NAAT) au Qatar
Au Qatar, le marché des tests d'amplification des acides nucléiques (TAAN) au point de service connaît une croissance constante grâce aux programmes de santé soutenus par le gouvernement et à l'importance croissante accordée au diagnostic préventif. Les hôpitaux et les cliniques adoptent les TAAN pour le dépistage des maladies infectieuses et la surveillance de routine. Le niveau élevé des dépenses de santé par habitant, associé à une infrastructure sanitaire solide, favorise l'adoption de diagnostics moléculaires avancés. L'utilisation du TAAN pour un diagnostic rapide aux urgences et en ambulatoire contribue également à l'expansion du marché. L'intégration aux systèmes informatiques hospitaliers permet un reporting en temps réel et une gestion efficace des patients. La sensibilisation croissante des patients aux diagnostics précoces encourage leur utilisation dans les secteurs de la santé, tant public que privé.
Part de marché des tests moléculaires au point de service (à l'aide du TAAN) au Moyen-Orient
L'industrie des tests moléculaires au point de service (utilisant le TAAN) au Moyen-Orient est principalement dirigée par des entreprises bien établies, notamment :
- Abbott (États-Unis)
- F. Hoffmann-La Roche SA (Suisse)
- BIOMÉRIEUX (France)
- QIAGEN (Pays-Bas)
- Danaher (États-Unis)
- Thermo Fisher Scientific Inc. (États-Unis)
- BD (États-Unis)
- Illumina, Inc. (États-Unis)
- Siemens Healthineers AG (Allemagne)
- Grifols, SA (Espagne)
- QuidelOrtho Corporation (États-Unis)
- DiaSorin SpA (Italie)
- Sysmex Corporation (Japon)
- Sekisui Diagnostics, LLC (États-Unis)
- Hologic, Inc. (États-Unis)
- Medtronic (Irlande)
- Atlas Medical GmbH (Allemagne)
- Nova Biomedical (États-Unis)
- Werfen (Espagne)
Quels sont les développements récents sur le marché des tests moléculaires au point de service (utilisant NAAT) au Moyen-Orient ?
- En février 2025, Aptitude a reçu une autorisation d'utilisation d'urgence (EUA) de la FDA américaine pour son test multiplex Metrix COVID/Grippe. Ce test offre une précision comparable à celle d'une PCR pour la détection et la différenciation du SARS-CoV-2, de la grippe A et de la grippe B en environ 20 minutes au chevet du patient.
- En janvier 2025, Roche a reçu l'autorisation de la FDA, avec dérogation CLIA, pour ses tests moléculaires cobas liat destinés au diagnostic des infections sexuellement transmissibles (IST) au chevet du patient. Ces tests, qui fournissent des résultats en 20 minutes, permettent aux cliniciens de diagnostiquer et de différencier plusieurs IST à partir d'un seul échantillon grâce à la technologie PCR de référence.
- En octobre 2024, Access Genetics (faisant affaire sous le nom d'OralDNA® Labs) a acquis Sensible Diagnostics Inc. L'objectif de l'acquisition est de commercialiser une nouvelle plateforme de diagnostic moléculaire au point de service qui vise à fournir des résultats de tests de qualité laboratoire central à partir de salive ou d'écouvillons en moins de 10 minutes, améliorant ainsi la rapidité et l'accessibilité des diagnostics.
- En septembre 2024, QIAGEN a élargi son partenariat stratégique avec l'entreprise brésilienne Bio-Manguinhos/Fiocruz. Cette collaboration vise à fournir des plateformes avancées de criblage moléculaire par PCR pour des maladies telles que le paludisme, le VIH et les hépatites B et C, dans le cadre du programme national brésilien de don du sang.
- En mai 2024, bioMérieux a annoncé un partenariat stratégique avec AnaBioTec, prestataire belge de services analytiques. Cette collaboration vise à transformer les tests Mycoplasma pour les industries biopharmaceutiques et de thérapie cellulaire et génique, en utilisant le système PCR entièrement automatisé de bioMérieux pour fournir une solution interne plus rapide et plus précise.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.