Middle East And Africa Car T Cell Therapy Treatment Market
Taille du marché en milliards USD
TCAC :
% 
 USD
16.22 Million
USD
138.11 Million
2024
2032
USD
16.22 Million
USD
138.11 Million
2024
2032
| 2025 –2032 | |
| USD 16.22 Million | |
| USD 138.11 Million | |
|
|
|
|
Segmentation du marché des thérapies cellulaires CAR-T MEA, par produit (cellules CAR-T autologues et allogéniques), structure (cellules CAR-T de première, deuxième, troisième et quatrième génération), antigènes ciblés (antigènes sur tumeurs solides, antigènes sur hémopathies malignes, etc.), marque (Yescarta, Kymriah, Tecartus, etc.), application thérapeutique (lymphome diffus à grandes cellules B, lymphome folliculaire, leucémie aiguë lymphoblastique (LAL), lymphome du manteau, myélome multiple, hémopathies malignes, cancer du poumon, leucémie lymphoïde chronique, cancer gastrique, cancer du pancréas, cancer du sein, etc.), utilisateur final (hôpitaux, cliniques spécialisées, etc.), canal de distribution (hôpitaux, pharmacies, etc.) - Tendances du secteur et prévisions jusqu'en 2032
Taille du marché des traitements par thérapie cellulaire CAR-T MEA
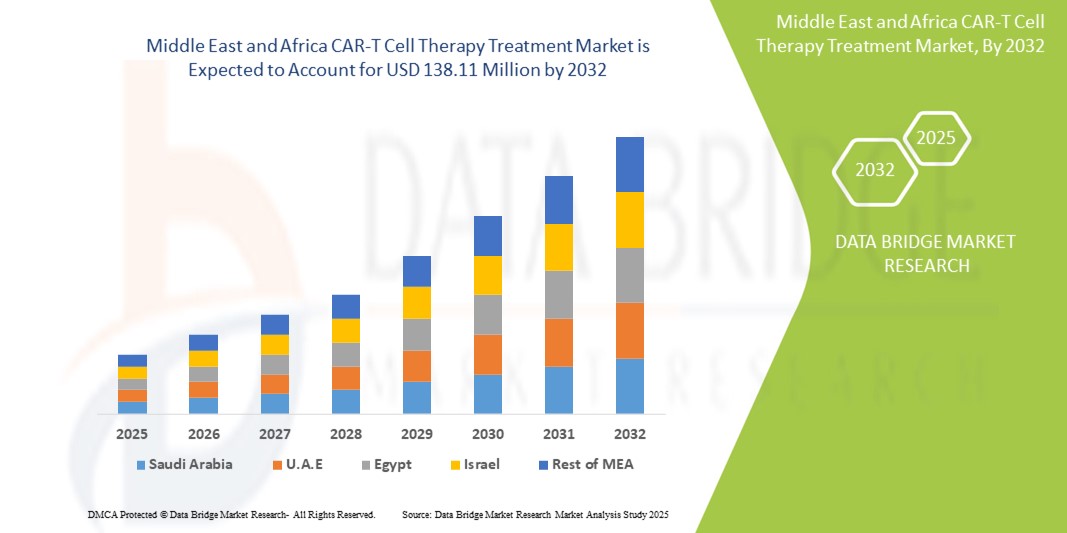
- La taille du marché du traitement par thérapie cellulaire CAR-T MEA était évaluée à 16,22 millions USD en 2024 et devrait atteindre 138,11 millions USD d'ici 2032 , à un TCAC de 30,7 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par la prévalence croissante des hémopathies malignes et l'expansion progressive des infrastructures de thérapie cellulaire et génique de pointe au Moyen-Orient et en Afrique (MOA). L'amélioration des cadres réglementaires, la croissance des investissements dans les biotechnologies et la création de centres de recherche et de fabrication de cellules CAR-T accélèrent le développement clinique et la disponibilité des thérapies cellulaires CAR-T.
- De plus, la demande croissante des patients pour des traitements ciblés, personnalisés et hautement efficaces positionne la thérapie cellulaire CAR-T comme une approche révolutionnaire dans la prise en charge du cancer. Ces facteurs convergents accélèrent l'adoption des solutions thérapeutiques CAR-T MEA, stimulant ainsi significativement la croissance du secteur sur des marchés clés tels que les Émirats arabes unis, l'Arabie saoudite, l'Afrique du Sud et l'Égypte.
Analyse du marché des thérapies cellulaires CAR-T MEA
- La thérapie cellulaire CAR-T, une immunothérapie innovante qui reprogramme les cellules T d'un patient pour reconnaître et attaquer les cellules cancéreuses, devient de plus en plus une approche thérapeutique essentielle pour les hémopathies malignes dans la région MEA en raison de sa grande efficacité, de sa nature personnalisée et de sa capacité à induire des rémissions durables dans les cas récidivants ou réfractaires.
- La demande croissante de thérapie cellulaire CAR-T est principalement alimentée par la prévalence croissante des cancers du sang tels que la leucémie et le lymphome, la sensibilisation croissante des prestataires de soins de santé et des patients et l'expansion de la recherche clinique dans la région MEA.
- Les Émirats arabes unis ont dominé le marché des traitements par thérapie cellulaire CAR-T MEA avec la plus grande part de revenus de 26,8 % en 2024, caractérisé par une solide infrastructure de soins de santé, une adoption précoce de traitements innovants et un investissement important dans les thérapies personnalisées contre le cancer.
- Israël devrait être le pays connaissant la croissance la plus rapide sur le marché des traitements par thérapie cellulaire CAR-T MEA avec un TCAC projeté de 17,5 % de 2025 à 2032, grâce à la recherche de pointe en immuno-oncologie, à un écosystème biotechnologique en expansion et à une forte activité d'essais cliniques en thérapie cellulaire et génique.
- Le segment des antigènes sur les hémopathies malignes a dominé le marché du traitement par thérapie cellulaire CAR-T MEA avec une part de marché de 66,4 % en 2024, alimenté par le succès des thérapies CAR-T pour les cancers du sang tels que la leucémie, le lymphome et le myélome multiple.
Portée du rapport et segmentation du marché des thérapies cellulaires CAR-T MEA
|
Attributs |
Informations clés sur le marché du traitement par thérapie cellulaire CAR-T MEA |
|
Segments couverts |
|
|
Pays couverts |
Moyen-Orient et Afrique
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des traitements par thérapie cellulaire CAR-T MEA
« Accès croissant aux immunothérapies avancées au Moyen-Orient et en Afrique »
- Une tendance significative et croissante sur le marché des thérapies cellulaires CAR-T en Afrique du Nord et en Afrique centrale est l'accès croissant aux immunothérapies avancées dans la région, notamment dans les centres de santé urbains. Les hôpitaux et cliniques spécialisées du Moyen-Orient et de certaines régions d'Afrique adoptent de plus en plus les thérapies cellulaires contre le cancer, témoignant d'une évolution vers la médecine de précision.
- Par exemple, plusieurs centres de cancérologie de pays comme les Émirats arabes unis (EAU) et l'Arabie saoudite se sont associés à des entreprises internationales de biotechnologie pour explorer les essais cliniques et les protocoles thérapeutiques des cellules CAR-T. Ces collaborations permettent aux médecins locaux de proposer des thérapies de pointe, jusqu'alors réservées aux marchés occidentaux.
- La prise de conscience régionale croissante des traitements oncologiques personnalisés incite les prestataires de soins de santé publics et privés à intégrer les CAR-T dans leurs stratégies de soins oncologiques à long terme. L'intensification des campagnes de sensibilisation et de défense des patients autour des hémopathies malignes contribue également à la croissance du marché.
- Par ailleurs, la région Moyen-Orient et Afrique (MEA) connaît une augmentation des initiatives gouvernementales visant à améliorer les taux de survie au cancer, telles que les programmes nationaux de lutte contre le cancer et les infrastructures de tests génomiques du cancer. Ces efforts sont essentiels pour identifier les patients éligibles à la thérapie cellulaire CAR-T et simplifier l'accès au traitement.
- Les centres médicaux universitaires de pays comme l'Afrique du Sud et l'Égypte explorent désormais des modèles de partenariat avec des sociétés biopharmaceutiques multinationales pour établir des centres de recherche et de fabrication de CAR-T, favorisant ainsi l'accessibilité régionale de la thérapie.
- La convergence de l'incidence croissante du cancer, du développement des infrastructures médicales et du soutien réglementaire crée un environnement favorable à l'expansion du marché de la thérapie CAR-T au Moyen-Orient et en Afrique.
Dynamique du marché des traitements par thérapie cellulaire CAR-T MEA
Conducteur
« Besoin croissant en raison de la charge croissante du cancer et des progrès de la médecine de précision »
- La prévalence croissante des hémopathies malignes, telles que le lymphome diffus à grandes cellules B, le myélome multiple et la leucémie aiguë lymphoblastique au Moyen-Orient et en Afrique (MEA), explique en grande partie la demande croissante de thérapies cellulaires CAR-T. L'augmentation de l'incidence du cancer et le besoin non satisfait de traitements efficaces et durables incitent les systèmes de santé à adopter des immunothérapies de pointe.
- Par exemple, en mars 2024, des prestataires de soins de santé régionaux d'Arabie saoudite et des Émirats arabes unis ont collaboré avec des entreprises mondiales de biotechnologie pour créer des centres de thérapie CAR-T, en mettant l'accent sur l'amélioration de l'accessibilité et des résultats pour les patients. Ces initiatives reflètent l'engagement stratégique de la région à intégrer les thérapies cellulaires avancées dans les parcours nationaux de soins contre le cancer.
- Par ailleurs, la région Moyen-Orient et Afrique (MOA) connaît une hausse des investissements dans la médecine personnalisée, favorisant l'application de thérapies CAR-T adaptées au profil tumoral de chaque patient. La disponibilité de produits CAR-T autologues comme Yescarta et Kymriah contribue à leur adoption précoce dans les hôpitaux spécialisés et les instituts de recherche.
- L'augmentation des budgets publics de santé et des cadres réglementaires favorables, notamment dans les pays du CCG, facilitent les essais cliniques et les autorisations de mise sur le marché des produits CAR-T. La sensibilisation croissante des oncologues et des patients à l'efficacité de ces thérapies en cas de rechute ou de récidive favorise également l'expansion du marché.
Retenue/Défi
« Coûts de traitement élevés et infrastructures limitées dans les marchés émergents »
- L'un des principaux défis qui freinent le marché des thérapies cellulaires CAR-T MEA est leur coût élevé, qui peut varier de 350 000 à 500 000 dollars par cycle de traitement. Ce coût comprend la récolte des cellules, la fabrication, les soins hospitaliers et le suivi, ce qui représente une charge importante pour les systèmes de santé publics et les patients non assurés.
- Par exemple, plusieurs pays africains manquent actuellement d'infrastructures de production et de stockage de cellules CAR-T, ce qui entraîne une dépendance à la logistique internationale et des délais de livraison prolongés. Cela affecte l'administration rapide du traitement et compromet les résultats, en particulier dans les cancers agressifs.
- De plus, le manque de personnel clinique qualifié et d'installations certifiées pour administrer la thérapie CAR-T constitue un obstacle à un accès généralisé. Les capacités diagnostiques limitées pour identifier les patients éligibles retardent encore davantage l'instauration du traitement.
- Pour surmonter ces obstacles, les acteurs du marché se concentrent sur le développement d'unités de production décentralisées de cellules CAR-T et sur la mise en place de programmes de formation en collaboration avec les gouvernements régionaux. Les efforts visant à réduire les coûts de production grâce à des plateformes de cellules CAR-T allogéniques (prêtes à l'emploi) pourraient également améliorer l'accessibilité financière dans les pays à revenu faible et intermédiaire de la région Moyen-Orient et Afrique.
- Relever les défis de remboursement, les complexités réglementaires et les contraintes logistiques grâce à des partenariats régionaux et des modèles de tarification innovants sera essentiel pour le succès à long terme du marché du traitement par thérapie cellulaire CAR-T MEA.
Portée du marché des traitements par thérapie cellulaire CAR-T MEA
Le marché du traitement par thérapie cellulaire CAR-T MEA est segmenté en quatre segments notables basés sur le produit, la structure, les antigènes ciblés et l'application thérapeutique.
• Par produit
Sur la base du produit, le marché de la thérapie cellulaire CAR-T MEA est segmenté en cellules CAR-T autologues et cellules CAR-T allogéniques. Le segment des cellules CAR-T autologues a dominé le marché avec la plus grande part de chiffre d'affaires (72,3 %) en 2024, grâce à une immunogénicité réduite et aux avantages de la personnalisation.
Le segment des cellules CAR-T allogéniques devrait connaître le TCAC le plus rapide de 24,8 % entre 2025 et 2032 en raison de leur disponibilité et de leur rentabilité.
• Par structure
Sur la base de sa structure, le marché des thérapies cellulaires CAR-T MEA est segmenté en cellules CAR-T de première génération, de deuxième génération, de troisième génération et de quatrième génération. Le segment des cellules CAR-T de deuxième génération détenait la plus grande part de marché, soit 58,9 % en 2024, en raison de leur efficacité accrue et de leur large application clinique.
Le segment des cellules CAR-T de quatrième génération devrait connaître le TCAC le plus rapide de 26,1 % au cours de la période de prévision, grâce aux technologies avancées d'édition de gènes et aux capacités de multi-ciblage.
• Par antigènes ciblés
Sur la base d'antigènes ciblés, le marché des thérapies cellulaires CAR-T MEA est segmenté en antigènes pour les tumeurs solides, antigènes pour les hémopathies malignes, etc. Les antigènes pour les hémopathies malignes ont représenté la plus grande part de chiffre d'affaires en 2024, avec 66,4 %, grâce au succès des thérapies CAR-T pour les cancers du sang.
Les antigènes présents sur les tumeurs solides devraient croître au TCAC le plus élevé de 25,7 % au cours de la période de prévision, grâce aux essais cliniques en cours et aux innovations dans le ciblage du microenvironnement tumoral.
• Par marque
Sur la base des marques, le marché des thérapies cellulaires CAR-T MEA est segmenté entre Yescarta, Kymriah et Tecartus, entre autres. Yescarta détenait la plus grande part de marché en 2024, avec 41,2 %, et a été fortement adopté pour le LDGCB et d'autres lymphomes.
Tecartus devrait enregistrer le TCAC le plus rapide de 22,9 % entre 2025 et 2032, grâce à ses performances dans le lymphome à cellules du manteau.
• Par application thérapeutique
En fonction de l'application thérapeutique, le marché des thérapies cellulaires CAR-T MEA est segmenté en lymphome diffus à grandes cellules B, lymphome folliculaire, leucémie aiguë lymphoblastique (LAL), lymphome à cellules du manteau, myélome multiple, hémopathies malignes, cancer du poumon, leucémie lymphoïde chronique, cancer gastrique, cancer du pancréas, cancer du sein, etc. Le lymphome diffus à grandes cellules B (LDGCB) a représenté la plus grande part de marché en 2024, avec 36,5 %, grâce à des autorisations précoces et à des résultats positifs pour les patients.
Le myélome multiple devrait croître au TCAC le plus rapide de 28,3 % au cours de la période de prévision, stimulé par les lancements de nouveaux produits CAR-T ciblant le BCMA au Moyen-Orient et en Afrique.
• Par l'utilisateur final
En fonction de l'utilisateur final, le marché des thérapies cellulaires CAR-T MEA est segmenté entre hôpitaux, cliniques spécialisées et autres. Les hôpitaux ont dominé ce segment avec une part de marché de 69,8 % en 2024, grâce à leur capacité à prendre en charge les protocoles de perfusion, de surveillance et de récupération des cellules CAR-T.
Les cliniques spécialisées devraient connaître une croissance au TCAC le plus élevé de 21,6 % au cours de la période de prévision, bénéficiant de la décentralisation et de l'expansion des réseaux d'immunothérapie.
• Par canal de distribution
En fonction du canal de distribution, le marché des thérapies cellulaires CAR-T MEA est segmenté entre pharmacies hospitalières et autres. En 2024, ce segment détenait la plus grande part de marché, soit 78,1 %, en raison de la complexité de la chaîne du froid et des exigences de conformité.
Le segment des autres devrait croître à un TCAC de 19,5 % au cours de la période de prévision, car de plus en plus de fabricants de CAR-T rationalisent la distribution par le biais de prestataires logistiques spécialisés.
Analyse régionale du marché des thérapies cellulaires CAR-T MEA
- Les Émirats arabes unis ont dominé le marché des traitements par thérapie cellulaire CAR-T MEA avec la plus grande part de revenus de 26,8 % en 2024, caractérisé par une solide infrastructure de soins de santé, une adoption précoce de traitements innovants et un investissement important dans les thérapies personnalisées contre le cancer.
- Les patients et les prestataires de soins de santé de la région apprécient de plus en plus l'efficacité ciblée, l'approche personnalisée et le potentiel de rémission à long terme offerts par les thérapies cellulaires CAR-T dans le traitement des cancers hématologiques tels que la leucémie et le lymphome.
- Cette adoption croissante est également stimulée par l'expansion des activités d'essais cliniques, l'amélioration de l'accès aux soins oncologiques avancés et l'augmentation des investissements gouvernementaux dans les traitements à base de cellules, positionnant la thérapie CAR-T comme une option transformatrice sur certains marchés du Moyen-Orient et de l'Afrique.
Aperçu du marché des thérapies cellulaires CAR-T MEA en Arabie saoudite
Le marché saoudien des thérapies cellulaires CAR-T représentait 21,3 % du chiffre d'affaires du marché Moyen-Orient et Afrique en 2024 et devrait connaître une croissance annuelle moyenne (TCAC) substantielle au cours de la période de prévision. Cette croissance est portée par la stratégie gouvernementale de transformation des soins de santé « Vision 2030 », l'expansion des centres d'oncologie spécialisés et les partenariats avec des entreprises internationales de biotechnologie. L'augmentation des cas de cancer hématologique et la volonté nationale d'adopter une médecine de précision stimulent l'expansion du marché.
Aperçu du marché des thérapies cellulaires CAR-T aux Émirats arabes unis (MEA)
Le marché des thérapies cellulaires CAR-T des Émirats arabes unis a enregistré la plus forte part de marché de la région Moyen-Orient et Afrique (MOA) en 2024, avec 26,8 %, grâce à des investissements importants dans les thérapies innovantes, des dépenses de santé élevées et des autorisations réglementaires proactives. Les thérapies CAR-T sont rapidement adoptées dans les principaux hôpitaux oncologiques du pays, grâce à des collaborations avec des innovateurs mondiaux tels que Gilead et Novartis.
Aperçu du marché israélien des thérapies cellulaires CAR-T MEA
Le marché israélien des thérapies cellulaires CAR-T devrait connaître un TCAC de 17,5 % entre 2025 et 2032, grâce à son secteur biotechnologique de pointe, à ses solides capacités de R&D universitaire et à son activité soutenue d'essais cliniques CAR-T. Ce marché est appelé à se développer davantage grâce au développement et à l'application croissants des immunothérapies de nouvelle génération au niveau national.
Part de marché du traitement par thérapie cellulaire CAR-T MEA
L'industrie du traitement par thérapie cellulaire CAR-T MEA est principalement dirigée par des entreprises bien établies, notamment :
- Novartis AG (Suisse)
- Gilead Sciences, Inc. (États-Unis)
- Bristol-Myers Squibb Company (États-Unis)
- Johnson & Johnson Services, Inc. (États-Unis)
- Autolus Therapeutics plc (Royaume-Uni)
- Poseida Therapeutics, Inc. (États-Unis)
- Sorrento Therapeutics, Inc. (États-Unis)
- Miltenyi Biotec (Allemagne)
- CARsgen Therapeutics (Chine)
- JW Therapeutics (Shanghai) Co., Ltd. (Chine)
- Legend Biotech Corporation (Chine)
- Tessa Therapeutics (Singapour)
- Adaptimmune Therapeutics plc (Royaume-Uni)
- Bluebird Bio, Inc. (États-Unis)
- Celyad Oncology SA (Belgique)
- Allogen Therapeutics (États-Unis)
- Immatics NV (Allemagne)
- Pfizer Inc. (États-Unis)
Dernières avancées sur le marché des thérapies cellulaires CAR-T MEA
- En décembre 2023, Gilead Sciences, Inc. a annoncé que la FDA américaine avait approuvé une mise à jour de l'étiquette de Yescarta (axicabtagène ciloleucel) afin d'inclure l'analyse principale de la survie globale (SG) de l'étude de phase 3 ZUMA-7, qui a montré une amélioration statistiquement significative de la SG pour Yescarta par rapport au traitement standard (SOC) en tant que traitement de deuxième intention à visée curative pour les patients atteints d'un lymphome à grandes cellules B récidivant ou réfractaire (LBCL R/R) dans les 12 mois suivant la fin du traitement de première intention.
- En mai 2022, Bristol-Myers Squibb a annoncé l'approbation d'Opdivo associé à Yervoy comme traitement de première intention chez les patients adultes par le ministère japonais de la Santé, du Travail et des Affaires sociales. Cette autorisation pourrait permettre à l'entreprise de renforcer son portefeuille de produits.
- En février 2022, la FDA a approuvé CARVYKTI (ciltacabtagène autoleucel) de Janssen pour le traitement des adultes atteints d'un myélome multiple récidivant ou réfractaire après quatre lignes de traitement ou plus. Il s'agit de la première thérapie cellulaire de Janssen. Cette initiative témoigne de la volonté de Janssen de faire progresser ses options thérapeutiques en oncologie.
- En décembre 2021, Novartis AG a signé un accord avec BeiGene, Ltd. pour l'ociperlimab (BGB-A1217), renforçant ainsi la recherche et le développement en immuno-oncologie de l'entreprise. Cette collaboration s'inscrit dans l'initiative plus vaste de Novartis Oncology visant à faire progresser l'innovation dans les traitements contre le cancer en intégrant une thérapie potentiellement transformatrice à sa plateforme d'immunothérapie en pleine expansion.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.