Global Pcr Based Transplant Diagnostics Market
Taille du marché en milliards USD
TCAC :
% 
 USD
652.31 Million
USD
1,079.57 Million
2024
2032
USD
652.31 Million
USD
1,079.57 Million
2024
2032
| 2025 –2032 | |
| USD 652.31 Million | |
| USD 1,079.57 Million | |
|
|
|
|
Segmentation du marché mondial des diagnostics de transplantation par PCR, par type de test (test PCR CMV, test PCR EBV, test PCR BKV, test PCR VZV, test PCR HSV1, test PCR HSV2, test PCR Parvovirus B19, test PCR P. Jirovecii, test PCR JCV, test PCR Adénovirus et test PCR Aspergillus Spp), type de transplantation (transplantation rénale, transplantation hépatique, transplantation cardiaque, transplantation pulmonaire, transplantation pancréatique et autres transplantations), application (applications diagnostiques et applications de recherche), utilisateur final (hôpitaux et centres de transplantation, prestataires de services commerciaux, laboratoires de recherche et instituts universitaires) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des diagnostics de transplantation basés sur la PCR
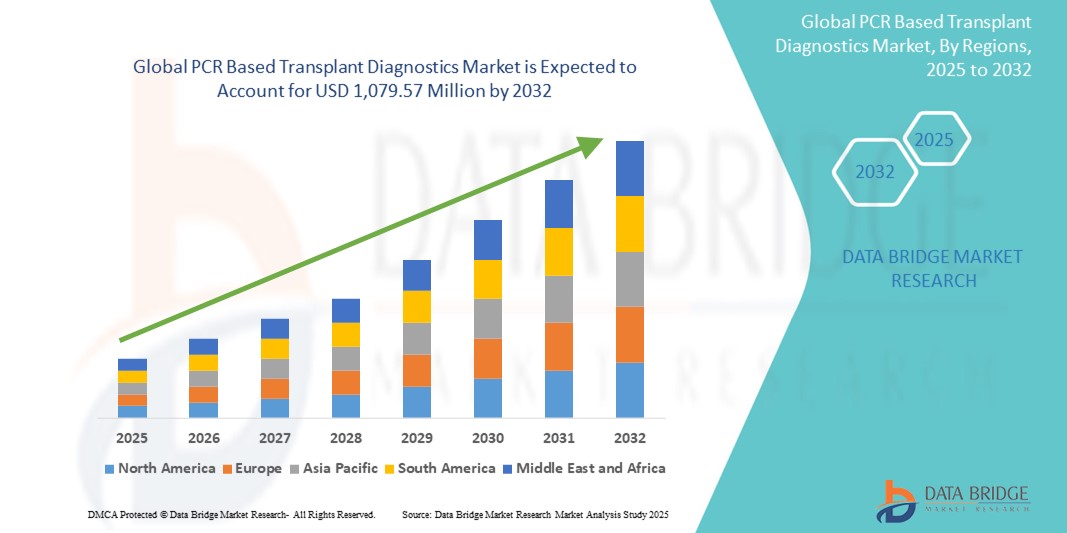
- La taille du marché mondial des diagnostics de transplantation basés sur la PCR était évaluée à 652,31 millions USD en 2024 et devrait atteindre 1 079,57 millions USD d'ici 2032 , à un TCAC de 6,50 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par l'adoption croissante et les progrès technologiques dans le domaine du diagnostic moléculaire et de la médecine de précision, conduisant à une numérisation et une précision accrues dans les procédures pré- et post-transplantation dans les hôpitaux et les laboratoires de diagnostic.
- De plus, la demande croissante de solutions de compatibilité de transplantation sûres, conviviales et fiables fait du diagnostic de transplantation par PCR la méthode privilégiée pour le typage HLA et la compatibilité donneur-receveur. Ces facteurs convergents accélèrent l'adoption de ces solutions, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché des diagnostics de transplantation basés sur la PCR
- Les diagnostics de transplantation par PCR, qui utilisent la technologie de réaction en chaîne par polymérase pour une compatibilité génétique de haute précision et la détection de la compatibilité donneur-receveur, deviennent de plus en plus essentiels pour améliorer les taux de réussite des transplantations et la détection précoce des rejets de greffe. Ces outils permettent un typage HLA sensible, spécifique et rapide, ainsi qu'un suivi post-transplantation, améliorant ainsi considérablement les résultats de la transplantation.
- La demande croissante de diagnostics de transplantation basés sur la PCR est principalement motivée par le nombre croissant de transplantations d'organes dans le monde, la prévalence croissante des maladies chroniques conduisant à une défaillance d'organe et l'adoption croissante des diagnostics moléculaires dans les laboratoires cliniques.
- L'Amérique du Nord a dominé le marché du diagnostic de transplantation par PCR, avec une part de chiffre d'affaires de 41,65 % en 2024, grâce à son infrastructure de santé avancée, à l'essor des procédures de transplantation d'organes et à l'adoption précoce des technologies de diagnostic moléculaire. Les États-Unis, en particulier, ont connu une croissance substantielle des innovations en matière de diagnostic de transplantation, soutenue par des politiques réglementaires favorables et d'importants investissements en R&D de la part des grandes entreprises de biotechnologie.
- L'Asie-Pacifique devrait connaître la croissance la plus rapide sur le marché des diagnostics de transplantation basés sur la PCR au cours de la période de prévision, grâce à l'élargissement de l'accès aux soins, à l'augmentation des volumes de transplantation et à la sensibilisation accrue à la médecine personnalisée dans des pays comme la Chine, l'Inde et le Japon. Les initiatives gouvernementales en faveur du don d'organes et de l'innovation diagnostique stimulent également la croissance régionale.
- Le segment des applications diagnostiques a dominé le marché des diagnostics de transplantation par PCR, avec une part de marché de 72,4 % en 2024, grâce à l'utilisation généralisée des tests PCR dans la prise en charge et le suivi systématiques des patients transplantés. Ces applications jouent un rôle crucial dans la détection précoce des infections virales, des risques de rejet de greffe et de la réponse au traitement, ce qui en fait des outils essentiels pour améliorer les résultats post-transplantation et la survie des patients.
Portée du rapport et segmentation du marché des diagnostics de transplantation basés sur la PCR
|
Attributs |
Informations clés sur le marché des diagnostics de transplantation basés sur la PCR |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
Europe
Asie-Pacifique
Moyen-Orient et Afrique
Amérique du Sud
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des diagnostics de transplantation basés sur la PCR
« Une commodité accrue grâce aux technologies moléculaires avancées et à l'automatisation »
- Une tendance significative et croissante sur le marché mondial du diagnostic de transplantation par PCR est l'intégration de technologies moléculaires avancées à des plateformes de diagnostic automatisées, permettant une détection plus rapide, plus précise et hautement sensible des infections et complications post-transplantation. Cette avancée améliore considérablement l'efficacité des flux de travail et la prise de décision clinique.
- Par exemple, les principales plateformes de diagnostic proposent désormais des tests PCR en temps réel pour détecter des virus tels que le CMV, l'EBV et le BKV, couramment surveillés chez les patients transplantés. Ces solutions permettent un dépistage à haut débit et une détection précoce, permettant ainsi une intervention rapide et améliorant les résultats de la transplantation.
- L'automatisation du diagnostic PCR réduit le risque d'erreur humaine, garantit des résultats cohérents et permet une surveillance continue de la charge virale chez les receveurs de greffe immunodéprimés. De nombreux systèmes prennent également en charge les tests multiplex, permettant la détection simultanée de plusieurs agents pathogènes lors d'un seul test.
- L'intégration transparente des outils de diagnostic PCR dans les systèmes d'information de laboratoire (SIL) et les dossiers médicaux électroniques (DME) des hôpitaux permet un flux de données rationalisé, un meilleur suivi de l'état des patients et des décisions de traitement plus éclairées.
- Cette tendance vers des systèmes de diagnostic plus intelligents et automatisés transforme fondamentalement les attentes en médecine de transplantation. Par conséquent, les entreprises développent en permanence des kits PCR avancés offrant une sensibilité accrue, des délais d'exécution plus courts et une couverture pathogène plus étendue, afin de répondre aux besoins changeants des hôpitaux, des centres de recherche et des laboratoires de transplantation.
- La demande de diagnostics de transplantation basés sur la PCR augmente rapidement dans les secteurs de la santé publique et privée, car les parties prenantes accordent la priorité à la détection précoce, à l'efficacité opérationnelle et à l'amélioration des soins aux patients dans les contextes de transplantation.
Dynamique du marché des diagnostics de transplantation basés sur la PCR
Conducteur
« Besoin croissant dû à l'augmentation des procédures de transplantation et aux avancées technologiques »
- L'augmentation mondiale des transplantations d'organes et de cellules souches, entraînée par le vieillissement de la population et la prévalence de maladies chroniques telles que l'insuffisance rénale et les cancers du sang, stimule considérablement la demande de diagnostics de transplantation précis et rapides basés sur la PCR.
- Par exemple, en février 2024, Eurofins Viracor a lancé un test PCR de nouvelle génération pour la détection précoce du virus BK chez les patients transplantés rénaux, visant à réduire le risque de rejet de greffe. Ces innovations technologiques devraient stimuler la croissance du marché en améliorant la précision du diagnostic et les délais d'exécution.
- La capacité des diagnostics PCR à fournir des résultats rapides, sensibles et spécifiques les rend essentiels à la fois pour les tests de compatibilité pré-transplantation et pour la surveillance post-transplantation, en particulier dans les cas critiques
- De plus, la sensibilisation accrue des cliniciens et des patients à l’importance de la détection précoce et de la surveillance immunitaire contribue à une adoption plus large des tests basés sur la PCR dans les contextes de transplantation.
- Les collaborations en cours entre les sociétés de diagnostic et les centres de transplantation, combinées à des investissements croissants dans le diagnostic moléculaire, créent un écosystème propice à une expansion rapide du marché, d'autant plus que la demande de soins de transplantation personnalisés s'intensifie.
Retenue/Défi
« Contraintes de coûts et défis de remboursement dans les milieux à faible revenu »
- Le coût relativement élevé des tests de diagnostic basés sur la PCR par rapport aux méthodes sérologiques traditionnelles constitue un obstacle, en particulier dans les pays en développement où les budgets de santé sont limités et l'accès aux tests moléculaires avancés est inégal.
- Par exemple, les centres de transplantation des économies émergentes sont souvent confrontés à des politiques de remboursement incohérentes pour les techniques de diagnostic avancées, notamment les tests basés sur la PCR, ce qui retarde leur adoption clinique généralisée.
- Les limitations des infrastructures, telles que le manque de laboratoires moléculaires avancés ou de personnel qualifié, entravent encore davantage le déploiement des tests PCR dans les environnements aux ressources limitées.
- Pour surmonter ces obstacles, il faut non seulement des réformes des prix et un soutien gouvernemental, mais aussi des partenariats public-privé plus importants pour développer les infrastructures de laboratoire et la formation des techniciens.
- En outre, l’harmonisation des cadres réglementaires mondiaux et des politiques de remboursement des assurances sera essentielle pour exploiter pleinement le potentiel des diagnostics de transplantation basés sur la PCR et promouvoir un accès équitable sur divers marchés.
Portée du marché des diagnostics de transplantation basés sur la PCR
Le marché est segmenté en fonction du type de test, du type de transplantation, de l’application et de l’utilisateur final.
- Par type de test
En fonction du type de test, le marché du diagnostic de transplantation par PCR est segmenté en tests PCR CMV, PCR EBV, PCR BKV, PCR VZV, PCR HSV1, PCR HSV2, PCR Parvovirus B19, PCR P. Jirovecii, PCR JCV, PCR Adénovirus et PCR Aspergillus Spp. Le segment des tests PCR CMV a dominé le marché avec la plus grande part de chiffre d'affaires (28,7 %) en 2024, en raison du besoin crucial de surveillance du CMV chez les receveurs de greffes d'organes solides et de cellules souches hématopoïétiques.
Le segment des tests PCR BKV devrait croître au TCAC le plus élevé de 16,2 % entre 2025 et 2032, alimenté par une sensibilisation croissante à la néphropathie BKV chez les patients transplantés rénaux et par l'utilisation croissante des diagnostics PCR quantitatifs.
- Par type de greffe
En fonction du type de transplantation, le marché est segmenté en transplantations rénales, hépatiques, cardiaques, pulmonaires et pancréatiques, entre autres. En 2024, la transplantation rénale détenait la plus grande part de marché, soit 39,5 %, grâce au volume mondial élevé de transplantations rénales et à l'utilisation systématique du diagnostic par PCR pour le suivi postopératoire.
Le segment de la transplantation cardiaque devrait connaître une croissance annuelle composée (TCAC) rapide de 14,3 % entre 2025 et 2032, en raison du nombre croissant de procédures et du besoin critique de surveillance des infections et d’évaluation de la réponse immunitaire.
- Par application
En fonction des applications, le marché du diagnostic de transplantation par PCR est segmenté en applications diagnostiques et applications de recherche. Le segment des applications diagnostiques a représenté la plus grande part de chiffre d'affaires, soit 72,4 % en 2024, grâce à l'utilisation généralisée des tests PCR dans la gestion et le suivi systématiques des patients transplantés.
Le segment des applications de recherche devrait connaître le TCAC le plus rapide, soit 13,6 %, au cours de la période de prévision, grâce à l'augmentation des investissements en R&D et aux études cliniques ciblant les voies de rejet et d'infection des greffes.
- Par utilisateur final
En fonction de l'utilisateur final, le marché est segmenté en hôpitaux et centres de transplantation, prestataires de services commerciaux, laboratoires de recherche et instituts universitaires. En 2024, le segment des hôpitaux et centres de transplantation a dominé le marché avec une part de marché record de 46,8 %, en raison de leur rôle essentiel dans le suivi post-transplantation et les soins directs aux patients.
Le segment des prestataires de services commerciaux devrait connaître le TCAC le plus rapide de 15,1 % entre 2025 et 2032, à mesure que l'externalisation du diagnostic et les services de tests spécialisés gagnent du terrain sur les marchés développés et émergents.
Analyse régionale du marché des diagnostics de transplantation basés sur la PCR
- L'Amérique du Nord a dominé le marché des diagnostics de transplantation par PCR, avec une part de chiffre d'affaires de 41,65 % en 2024, grâce à la prévalence croissante des maladies chroniques, au volume élevé de transplantations d'organes et à l'adoption précoce de diagnostics moléculaires avancés. La région bénéficie d'infrastructures de santé bien établies, de politiques de remboursement avantageuses et d'une forte présence d'acteurs clés proposant des solutions innovantes de tests PCR.
- L'importance croissante accordée à la médecine de précision et aux soins post-transplantation personnalisés renforce la domination de la région. Les avancées technologiques des plateformes PCR et l'approbation réglementaire de nouveaux diagnostics de transplantation accélèrent également la croissance du marché en Amérique du Nord.
- De plus, la sensibilisation croissante des cliniciens et des patients à l’importance de la détection précoce du rejet de greffe et de la surveillance virale après la transplantation alimente la demande de méthodes de test précises et rapides basées sur la PCR.
Aperçu du marché américain des diagnostics de transplantation basés sur la PCR
En 2024, le marché américain du diagnostic de transplantation basé sur la PCR a représenté la plus grande part de chiffre d'affaires en Amérique du Nord, soit 68 %, grâce au taux élevé de transplantations d'organes solides et de moelle osseuse et à un paysage diagnostique technologiquement avancé. La présence d'entreprises de diagnostic de premier plan, ainsi qu'un financement de la recherche solide et des voies réglementaires favorables, favorisent l'innovation et l'accessibilité. Des initiatives clés d'organisations nationales, telles que l'American Society of Transplantation, favorisent l'utilisation du diagnostic moléculaire pour améliorer les résultats des transplantations.
Analyse du marché européen des diagnostics de transplantation basés sur la PCR
Le marché européen des diagnostics de transplantation par PCR devrait connaître une croissance annuelle composée (TCAC) substantielle au cours de la période de prévision, portée par l'augmentation des dépenses de santé, le développement des systèmes de santé et le besoin croissant de suivi post-transplantation. L'accent mis par la région sur l'adoption de diagnostics moléculaires innovants pour la surveillance de la charge virale (par exemple, CMV, EBV) et des marqueurs de rejet chez les patients transplantés contribue significativement à la croissance du marché. Ce marché bénéficie également d'un soutien gouvernemental important et de collaborations en recherche clinique entre des pays comme l'Allemagne, la France et le Royaume-Uni.
Aperçu du marché britannique des diagnostics de transplantation basés sur la PCR
Le marché britannique des diagnostics de transplantation basés sur la PCR devrait connaître une croissance annuelle moyenne (TCAC) remarquable, grâce au renforcement des programmes nationaux de transplantation et à la demande croissante de diagnostics de transplantation non invasifs et hautement sensibles. L'adoption des tests de typage HLA et de surveillance virale basés sur la PCR est en hausse dans les laboratoires publics et privés. L'accent stratégique mis par le NHS sur l'amélioration des résultats des transplantations et la réduction des délais d'attente devrait également stimuler la demande pour ces diagnostics.
Analyse du marché allemand des diagnostics de transplantation basés sur la PCR
Le marché allemand du diagnostic de transplantation par PCR devrait connaître une croissance considérable au cours de la période de prévision, grâce à sa solide infrastructure de recherche clinique, à son taux élevé de transplantations et à l'adoption rapide des tests moléculaires. Ce marché est soutenu par des investissements proactifs dans la pathologie numérique et la génomique, et les laboratoires allemands comptent parmi les premiers à adopter les systèmes de PCR en temps réel pour la compatibilité donneur-receveur et le suivi post-transplantation.
Analyse du marché des diagnostics de transplantation basés sur la PCR en Asie-Pacifique
Le marché du diagnostic de transplantation basé sur la PCR en Asie-Pacifique devrait connaître son taux de croissance annuel composé (TCAC) le plus élevé, soit 24 % entre 2025 et 2032, grâce à la hausse des dépenses de santé, à la hausse des taux de transplantation et à l'amélioration des infrastructures de laboratoire dans des pays comme la Chine, le Japon et l'Inde. La sensibilisation croissante aux complications de la transplantation et à l'importance d'une détection précoce grâce au diagnostic par PCR stimule la demande. De plus, les initiatives de don d'organes soutenues par les gouvernements et les collaborations avec des entreprises mondiales de diagnostic ouvrent de nouvelles perspectives de croissance dans la région.
Analyse du marché japonais des diagnostics de transplantation basés sur la PCR
Le marché japonais du diagnostic de transplantation par PCR connaît un essor important en raison du vieillissement de la population, des avancées technologiques en matière de santé et de l'importance accordée à la médecine personnalisée. Les méthodes de diagnostic par PCR sont de plus en plus utilisées dans les centres de transplantation pour détecter les infections virales et la compatibilité donneur-receveur avec une grande précision. Le soutien de l'assurance maladie nationale et l'intégration de plateformes moléculaires automatisées facilitent l'accès au diagnostic dans les principaux hôpitaux et services de transplantation.
Analyse du marché chinois des diagnostics de transplantation basés sur la PCR
En 2024, le marché chinois des diagnostics de transplantation par PCR représentait la plus grande part de marché en Asie-Pacifique, grâce à l'urbanisation rapide, à l'augmentation des volumes de transplantation et aux investissements publics dans la modernisation des soins de santé. Les entreprises de diagnostic locales et internationales renforcent leur présence pour répondre à la demande de tests de charge virale par PCR, de typage tissulaire et de suivi des réponses immunitaires post-transplantation.
La poussée vers la numérisation des soins de santé et l'amélioration des capacités des laboratoires
Part de marché des diagnostics de transplantation basés sur la PCR
L'industrie du diagnostic de transplantation basé sur la PCR est principalement dirigée par des entreprises bien établies, notamment :
- Quest Diagnostics Incorporated
- Laboratoire Corporation of America Holdings (Labcorp)
- Eurofins Viracor, LLC
- Sonic Healthcare États-Unis
- Laboratoires ARUP
- Laboratoire Ambar
- Dr Lal PathLabs
Derniers développements sur le marché mondial des diagnostics de transplantation basés sur la PCR
- En juin 2025 , Insight Molecular Diagnostics (iMDx) a annoncé une étude phare comparant son test GraftAssureIQ basé sur la PCR numérique à un test commercial basé sur la NGS chez 96 patients transplantés rénaux . L'étude, menée à l'hôpital universitaire de Heidelberg, a révélé des résultats cliniquement équivalents , tandis que la PCR numérique a montré une sensibilité supérieure pour la détection de faibles concentrations d'ADN acellulaire dérivé du donneur (dd-cfDNA).
- En janvier 2024, Laboratory Corporation of America Holdings a lancé un nouveau test de dépistage de la prééclampsie chez la femme enceinte, ce qui représente une avancée significative dans les soins maternels. Grâce à ces outils de diagnostic avancés, les professionnels de santé peuvent identifier les femmes présentant un risque accru de prééclampsie sévère plus tôt dans leur grossesse. Cela permet à l'entreprise d'assurer une surveillance plus vigilante et une intervention médicale proactive, réduisant potentiellement le risque de complications et améliorant les résultats pour la mère et le fœtus.
- En septembre 2023, Sonic Healthcare a acquis Healius Ltd. afin de se développer dans le secteur australien de la santé. Cette acquisition renforce la position de Sonic en tant que leader mondial de la pathologie et renforce son avantage concurrentiel face aux autres acteurs internationaux. Elle témoigne de la volonté de l'entreprise de se développer et de se consolider au sein du secteur, ouvrant potentiellement la voie à des fusions similaires dans d'autres régions.
- En octobre 2023, Sonic Healthcare a investi dans PaigeAI, une entreprise de pathologie basée sur l'IA, soulignant ainsi son engagement à exploiter les technologies de pointe pour améliorer la précision et l'efficacité des diagnostics en pathologie. Cette acquisition a des implications importantes pour l'avenir du diagnostic médical. Cette approche de précision peut conduire à des traitements plus efficaces et potentiellement réduire le risque d'effets secondaires liés à des médicaments inutiles.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.