Global Influenza Drug Market
Taille du marché en milliards USD
TCAC :
% 
 USD
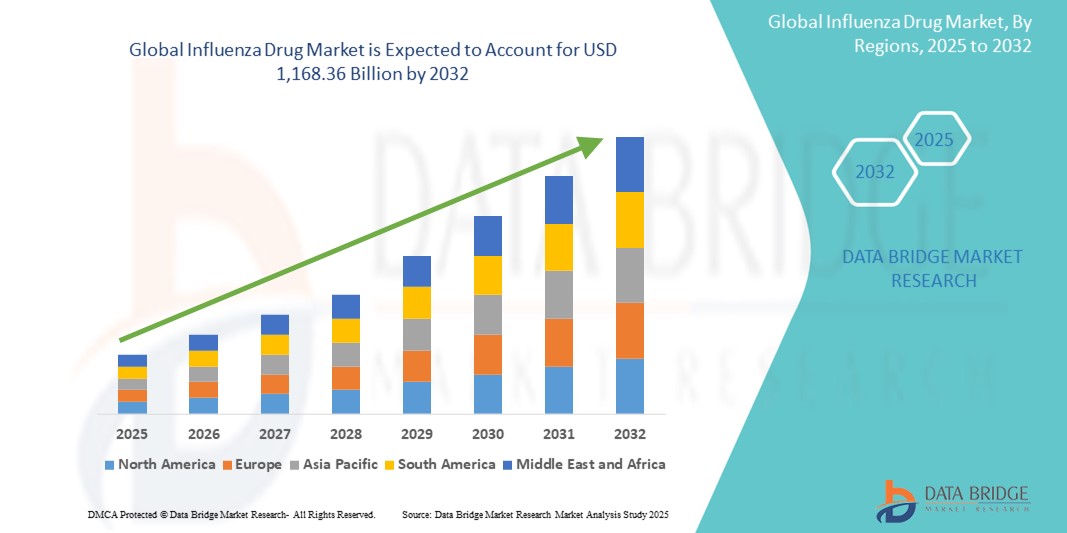
981.68 Billion
USD
1,168.36 Billion
2024
2032
USD
981.68 Billion
USD
1,168.36 Billion
2024
2032
| 2025 –2032 | |
| USD 981.68 Billion | |
| USD 1,168.36 Billion | |
|
|
|
|
Segmentation du marché mondial des médicaments contre la grippe, par type (grippe A, grippe B et grippe C), traitement (vaccins et médicaments), voie d'administration (orale, intramusculaire, intradermique, intranasale et intraveineuse), âge (pédiatrie et adultes), utilisateur final (hôpitaux et soins à domicile), canal de distribution (appels d'offres directs et vente au détail) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des médicaments contre la grippe
- La taille du marché mondial des médicaments contre la grippe était évaluée à 981,68 milliards USD en 2024 et devrait atteindre 1 168,36 milliards USD d'ici 2032 , à un TCAC de 2,20 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par l'incidence croissante de la grippe saisonnière et d'autres maladies respiratoires contagieuses, ainsi que par une population croissante de patients risquant de développer des complications liées à la grippe.
- En outre, l'augmentation des investissements dans les activités de recherche et développement par les sociétés pharmaceutiques et les instituts de recherche pour développer des médicaments avancés et améliorés, y compris de nouveaux médicaments antiviraux et des vaccins recombinants , accélère l'adoption de solutions médicamenteuses contre la grippe, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché des médicaments contre la grippe
- Le marché mondial des médicaments contre la grippe comprend une gamme de médicaments antiviraux et de vaccins conçus pour prévenir, traiter et soulager les symptômes des infections grippales, qui posent un défi de santé publique important en raison de leur nature récurrente et saisonnière, de leurs mutations virales constantes et de leur potentiel pandémique.
- La demande croissante de médicaments contre la grippe est principalement alimentée par la prévalence mondiale constante des infections grippales, la sensibilisation croissante aux avantages de la vaccination et les progrès continus de la recherche pharmaceutique conduisant à des thérapies antivirales plus efficaces et à des vaccins de nouvelle génération.
- L'Amérique du Nord domine le marché des médicaments contre la grippe, avec une part de marché de 60,5 % en 2024, et se caractérise par une infrastructure de santé robuste, une couverture vaccinale élevée et la présence de grandes entreprises pharmaceutiques. Les États-Unis connaissent une forte croissance du marché, portée par de vastes campagnes de santé publique, des initiatives gouvernementales de préparation aux pandémies et des efforts continus de recherche et développement sur de nouveaux traitements et vaccins contre la grippe.
- L'Asie-Pacifique devrait être la région connaissant la croissance la plus rapide sur le marché des médicaments contre la grippe au cours de la période de prévision en raison de la sensibilisation croissante à la grippe, de l'augmentation des dépenses de santé, de l'urbanisation croissante et d'un large bassin de patients sensibles aux infections dans les pays densément peuplés.
- Le segment de la grippe A domine le marché des médicaments contre la grippe avec une part de marché de 47,78 % en 2024, en raison de son taux de mutation plus élevé, de sa gamme d'hôtes plus large, de son potentiel pandémique important et de la gravité accrue de la maladie, nécessitant des stratégies de vaccination plus complexes et des mises à jour fréquentes des traitements.
Portée du rapport et segmentation du marché des médicaments contre la grippe
|
Attributs |
Aperçu du marché des médicaments contre la grippe |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
Europe
Asie-Pacifique
Moyen-Orient et Afrique
Amérique du Sud
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des médicaments contre la grippe
« Adoption croissante de la télémédecine et des outils de santé numériques »
- Une tendance significative et croissante sur le marché mondial des médicaments contre la grippe est l'intégration croissante de la télémédecine et des outils de santé numériques pour la gestion, le diagnostic et la prescription des médicaments contre la grippe. Cette fusion des technologies améliore considérablement l'accessibilité aux services de santé, notamment pour les patients atteints de grippe.
- Par exemple, les consultations virtuelles permettent aux personnes présentant des symptômes grippaux de contacter des professionnels de santé depuis leur domicile, réduisant ainsi le risque de transmission virale en clinique et à l'hôpital. Les plateformes de télémédecine peuvent faciliter un diagnostic rapide basé sur l'évaluation des symptômes et orienter les patients vers les soins auto-administrés appropriés ou la prescription de médicaments antiviraux tels que l'oseltamivir ou le baloxavir. Environ 33 % des systèmes de santé intègrent le traitement de la grippe aux services de télémédecine, améliorant ainsi l'accessibilité dans les régions rurales.
- Les outils de santé numériques, notamment les applications mobiles et les objets connectés, permettent une surveillance continue des paramètres physiologiques pouvant indiquer l'apparition ou la progression de la grippe. Certains thermomètres intelligents , comme ceux de KINSA, peuvent recueillir des données de température agrégées afin d'identifier les foyers grippaux potentiels. Ces technologies peuvent fournir des alertes précoces, incitant les personnes à consulter un médecin plus rapidement, ce qui est crucial pour l'efficacité des traitements antiviraux.
- L'intégration harmonieuse des outils de santé numérique aux systèmes de santé favorise également des efforts plus larges de surveillance de la santé publique. Les données issues des appareils portables peuvent contribuer à la surveillance de la grippe en temps réel, aidant ainsi les autorités sanitaires à suivre les épidémies et à allouer les ressources plus efficacement. Cette surveillance renforcée peut éclairer les campagnes de vaccination ciblées et la distribution rapide des médicaments antiviraux.
- Cette tendance vers une gestion de la grippe plus accessible, proactive et basée sur les données transforme en profondeur l'engagement des patients et la prestation des soins. Par conséquent, les laboratoires pharmaceutiques et les professionnels de santé explorent des partenariats et développent des plateformes exploitant ces capacités numériques pour améliorer les résultats des patients et l'observance thérapeutique.
- La demande de médicaments contre la grippe est indirectement stimulée par ces tendances, car la télémédecine permet aux patients de recevoir plus facilement des ordonnances en temps opportun, et la surveillance numérique peut conduire à un diagnostic et une intervention plus précoces, maximisant ainsi l'efficacité des traitements disponibles.
Dynamique du marché des médicaments contre la grippe
Conducteur
« Besoin croissant en raison de la prévalence mondiale constante de la grippe et des initiatives de santé publique »
- La prévalence mondiale croissante et constante des épidémies de grippe saisonnière, associée à la menace persistante de souches pandémiques et à des initiatives de santé publique robustes, constitue un facteur important de la demande accrue de médicaments et de vaccins contre la grippe.
- Par exemple, les gouvernements du monde entier, notamment aux États-Unis, en Europe et en Asie, lancent continuellement de vastes campagnes de vaccination et investissent dans des programmes nationaux de vaccination afin d'atténuer l'impact des grippes saisonnières et de se préparer à d'éventuelles pandémies. Ces initiatives, associées à une surveillance accrue et à des progrès rapides en matière de diagnostic, favorisent la vaccination et le traitement précoce par antiviraux.
- La sensibilisation croissante du grand public et des professionnels de santé à la gravité de la grippe et à ses complications potentielles accroît la demande de solutions préventives et thérapeutiques. Cela est particulièrement vrai pour les populations à risque, comme les personnes âgées, les jeunes enfants et les personnes souffrant de problèmes de santé sous-jacents.
- En outre, les efforts continus de recherche et développement déployés par les sociétés pharmaceutiques pour créer des médicaments antiviraux plus efficaces et à spectre plus large, ainsi que des vaccins de nouvelle génération, élargissent le paysage thérapeutique disponible et améliorent l'efficacité des interventions existantes, propulsant ainsi la croissance du marché.
- La nécessité de mettre à jour chaque année les formulations de vaccins en raison de l'évolution constante des virus grippaux assure une demande soutenue et récurrente de nouveaux produits. Ce cycle continu de développement, de production et de distribution est un facteur clé de la croissance du marché des médicaments antigrippaux, tant dans les économies développées qu'émergentes.
Retenue/Défi
« Défis liés à la mutabilité virale et à la résistance aux médicaments, et coûts de développement élevés »
- Un défi majeur pour le marché mondial des médicaments antigrippaux réside dans la mutabilité inhérente des virus grippaux, qui subissent constamment des variations et des changements antigéniques. Cette évolution rapide peut réduire l'efficacité des vaccins existants et favoriser l'émergence de résistances aux antiviraux, constituant une menace permanente pour la santé publique.
- Par exemple, la nécessité fréquente de mettre à jour les souches vaccinales chaque année nécessite un cycle continu de recherche, de développement et de production, à la fois long et coûteux. De plus, l'émergence de souches pharmacorésistantes, telles que celles résistantes à d'anciens antiviraux comme l'amantadine ou, plus récemment, à certains virus H1N1 résistants à l'oseltamivir (Tamiflu), limite les options thérapeutiques et complique la prise en charge clinique.
- Relever ces défis biologiques nécessite des investissements importants et soutenus en R&D pour développer de nouveaux agents antiviraux dotés de mécanismes d'action inédits et d'un spectre d'activité plus large, ainsi que des vaccins antigrippaux universels offrant une protection durable contre de multiples souches. Cependant, le développement de vaccins et de médicaments est un processus exceptionnellement long et coûteux, prenant souvent 10 à 15 ans et coûtant des centaines de millions à plus d'un milliard de dollars, avec un taux d'échec élevé.
- De plus, les défis logistiques, notamment le maintien de la chaîne du froid pour la distribution des vaccins depuis les sites de production jusqu'aux zones reculées, peuvent entraîner des gaspillages et une efficacité réduite. Des facteurs tels que l'insuffisance des infrastructures, les erreurs humaines et les pannes de courant dans les pays à faible revenu constituent des obstacles importants.
- Le coût initial élevé du développement et de la mise sur le marché de nouveaux médicaments et vaccins contre la grippe, combiné à des processus d'approbation réglementaires rigoureux, crée des obstacles importants pour les fabricants. Cela se traduit souvent par des prix plus élevés pour les traitements avancés, ce qui peut limiter l'accès aux marchés sensibles aux prix ou aux populations sous-assurées.
- Surmonter ces défis grâce à des collaborations internationales, un financement public et privé accru pour la R&D, des voies réglementaires simplifiées et des investissements dans des infrastructures de distribution mondiales robustes sera essentiel pour une croissance soutenue du marché et une préparation efficace aux pandémies.
Portée du marché des médicaments contre la grippe
Le marché est segmenté en fonction du type, du traitement, de la voie d’administration, de l’âge, de l’utilisateur final et du canal de distribution.
- Par type
Le marché des médicaments antigrippaux est segmenté en fonction du type de virus : grippe A, grippe B et grippe C. Le segment de la grippe A domine le marché avec une part de marché de 47,78 % en 2024, grâce à son taux de mutation plus élevé, sa plus large gamme d'hôtes, son potentiel pandémique important et la gravité accrue de la maladie, nécessitant des stratégies de vaccination plus complexes pour contrôler les épidémies. La menace constante des souches de grippe A, telles que H1N1 et H3N2, assure une demande soutenue de médicaments et de vaccins associés.
La grippe B devrait connaître le TCAC le plus élevé du marché en raison de son inclusion dans les vaccins quadrivalents, qui offrent une protection plus étendue contre les souches A et B. La prise de conscience croissante du rôle de la grippe B dans les épidémies saisonnières et l'importance croissante accordée aux stratégies globales de prévention de la grippe stimulent la demande de vaccins plus efficaces couvrant ces souches, en particulier chez les populations vulnérables.
- Par traitement
En fonction du traitement, le marché des médicaments contre la grippe est segmenté en vaccins et en médicaments. Le segment des vaccins domine le marché avec une part de marché de 87,23 % en 2024, en raison de son rôle essentiel dans la prévention de la maladie, la réduction de la transmission et la réponse aux préoccupations de santé publique liées aux épidémies saisonnières et aux pandémies potentielles. Les progrès constants de la technologie vaccinale, notamment le développement de vaccins quadrivalents offrant une protection plus étendue, renforcent encore leur leadership sur le marché.
Le segment des médicaments, y compris les antiviraux tels que l'oseltamivir et le baloxavir, joue un rôle crucial dans le traitement des infections établies, le baloxavir marboxil (Xofluza) étant considéré comme le type de médicament qui connaît la croissance la plus rapide en raison de son administration à dose unique et de son efficacité.
- Par voie d'administration
Selon la voie d'administration, le marché des médicaments antigrippaux est segmenté en trois catégories : orale, intramusculaire, intradermique, intranasale et intraveineuse. La voie orale devrait dominer le marché avec une part de marché de 45,04 % en 2024, principalement grâce à sa praticité, sa facilité d'auto-administration et son accessibilité généralisée pour les patients, tant en ambulatoire qu'à domicile. Les antiviraux oraux constituent souvent le traitement de première intention contre la grippe.
Le segment intranasal devrait être la voie d'administration connaissant la croissance la plus rapide au cours de la période de prévision. Cela est dû à son administration non invasive et sans aiguille, qui améliore l'observance du traitement, notamment chez les enfants, et à sa capacité à induire une immunité systémique et muqueuse.
- Par âge
En fonction de l'âge, le marché des médicaments antigrippaux est segmenté entre les enfants et les adultes. Le segment adulte est le plus important sur le marché antigrippal, avec une part de marché de 66,7 % en 2024, grâce à des taux de vaccination élevés chez les seniors et les personnes atteintes de maladies chroniques, qui présentent un risque accru de complications grippales graves.
Le segment pédiatrique est sur le point de croître à un TCAC notable, car les jeunes enfants, en particulier ceux de moins de 5 ans, présentent un risque plus élevé de complications grippales graves, ce qui accélère le champ d'application du marché des formulations adaptées aux enfants et des efforts de vaccination.
- Par utilisation finale
En fonction de l'utilisateur final, le marché des médicaments antigrippaux est segmenté entre les hôpitaux et les soins à domicile. Le segment hospitalier domine le marché mondial des médicaments antigrippaux avec une part de marché de 72,62 % en 2024, principalement en raison de son rôle dans la prise en charge des cas de grippe sévère nécessitant une surveillance intensive, des antiviraux par voie intraveineuse et des soins intensifs pour les patients à haut risque. Les hôpitaux constituent également des points clés pour les campagnes de vaccination.
Le segment des soins à domicile devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide du marché mondial des médicaments contre la grippe entre 2025 et 2032, grâce à la préférence croissante pour les traitements à domicile, aux progrès de la télémédecine et à l'adoption croissante des services de soins à domicile. Ces facteurs permettent aux patients de gérer efficacement les symptômes de la grippe dans le confort de leur domicile, réduisant ainsi le recours à l'hospitalisation et minimisant l'exposition aux établissements de santé.
- Par canal de distribution
En fonction du canal de distribution, le marché mondial des médicaments contre la grippe est segmenté en appels d'offres directs et ventes au détail. Le segment des appels d'offres directs devrait dominer le marché mondial des médicaments contre la grippe avec une part de marché de 56,34 % en 2024, principalement grâce aux achats massifs de vaccins et d'antiviraux par les gouvernements et les organismes de santé publique pour les programmes nationaux de vaccination et la constitution de stocks stratégiques. Ce canal garantit des achats en gros et une distribution efficace pour les campagnes de vaccination de masse.
Le segment des ventes au détail est également un canal dominant, servant de point d’accès clé pour les vaccins et les traitements en vente libre et sur ordonnance destinés au grand public, et devrait connaître une croissance significative, notamment grâce aux pharmacies en ligne en raison de leur commodité et de leur accessibilité.
Analyse régionale du marché des médicaments contre la grippe
- L'Amérique du Nord domine le marché des médicaments contre la grippe avec la plus grande part de revenus de 60,5 % en 2024, grâce à une infrastructure de soins de santé robuste, des taux de couverture vaccinale élevés et la présence de grandes sociétés pharmaceutiques.
- Les consommateurs de la région accordent une grande priorité aux soins de santé préventifs et accèdent facilement aux vaccins et aux traitements antiviraux
- Cette adoption généralisée est en outre soutenue par des politiques de remboursement favorables, une population technologiquement avancée et l'accent croissant mis sur les initiatives de santé publique, faisant des médicaments contre la grippe un élément crucial de la gestion des maladies saisonnières.
Aperçu du marché américain des médicaments contre la grippe
En 2024, le marché américain des médicaments contre la grippe a représenté la plus grande part de revenus en Amérique du Nord, avec 56,2 %, ce qui témoigne du système de santé avancé du pays et de ses stratégies proactives de santé publique. Les États-Unis sont un moteur majeur du marché de la grippe en raison de la charge importante de la grippe saisonnière, de programmes de vaccination robustes et d'investissements continus en R&D pour de nouveaux traitements et vaccins. Les consommateurs privilégient de plus en plus les mesures préventives et ont facilement accès aux médicaments contre la grippe. La forte intégration des recommandations de santé publique, la large disponibilité des vaccins et des antiviraux, ainsi qu'une importante population de patients contribuent à cette demande soutenue.
Aperçu du marché européen des médicaments contre la grippe
Le marché européen des médicaments antigrippaux devrait croître à un TCAC de 2,5 % sur la période de prévision, principalement grâce aux initiatives gouvernementales en matière de vaccination et de traitement de la grippe, aux directives strictes de santé publique et à la forte incidence de la grippe saisonnière. L'accent mis par la région sur les soins de santé préventifs, associé à des infrastructures de santé avancées et à une sensibilisation accrue aux complications de la grippe, favorise l'adoption de médicaments antigrippaux. Les pays européens connaissent une demande constante de vaccins et de traitements antiviraux en réponse aux épidémies annuelles.
Aperçu du marché britannique des médicaments contre la grippe
Le marché britannique des médicaments antigrippaux devrait connaître une croissance annuelle moyenne (TCAC) remarquable au cours de la période de prévision, portée par des programmes gouvernementaux de vaccination rigoureux, une attention croissante portée à la santé publique et la volonté d'une protection renforcée contre la grippe saisonnière. Le système de santé britannique, solidement établi, et les recommandations strictes de vaccination annuelle contre la grippe encouragent une forte utilisation des vaccins et des traitements antiviraux au sein de la population. L'engagement du pays en faveur des initiatives de santé publique stimule également la croissance du marché.
Aperçu du marché allemand des médicaments contre la grippe
Le marché allemand des médicaments antigrippaux devrait connaître une croissance annuelle moyenne (TCAC) considérable au cours de la période de prévision, alimentée par une sensibilisation croissante à la sécurité sanitaire, une forte importance accordée à la médecine préventive et une demande de solutions technologiques avancées. Le développement des infrastructures de santé allemandes, combiné à l'importance accordée à des taux de vaccination élevés et à une gestion efficace des maladies, favorise l'adoption de médicaments antigrippaux, notamment en médecine générale et en milieu hospitalier. L'intégration de diagnostics avancés et la préférence pour des traitements fondés sur des données probantes répondent aux attentes des consommateurs et des professionnels de la santé locaux.
Aperçu du marché des médicaments contre la grippe en Asie-Pacifique
Le marché des médicaments antigrippaux en Asie-Pacifique devrait connaître son taux de croissance annuel composé le plus élevé au cours de la période de prévision, porté par l'urbanisation croissante, la hausse des revenus disponibles et les avancées technologiques en matière de santé dans des pays comme la Chine, le Japon et l'Inde. La population nombreuse et croissante de la région est très vulnérable aux épidémies de grippe, ce qui entraîne une sensibilisation et une demande croissantes en traitements et vaccins efficaces. De plus, avec le développement des infrastructures de santé en Asie-Pacifique et les initiatives gouvernementales favorisant un accès plus large aux médicaments essentiels, l'accessibilité et l'abordabilité des médicaments antigrippaux s'étendent à un public plus large.
Aperçu du marché japonais des médicaments contre la grippe
Le marché japonais des médicaments antiviraux contre la grippe connaît une forte croissance, en raison de la forte incidence de la grippe saisonnière, de l'urbanisation rapide et de la forte demande de traitements efficaces. Le marché japonais accorde une importance majeure à la santé publique et à la couverture vaccinale, et l'adoption de médicaments antigrippaux est stimulée par une prise de conscience croissante des complications, notamment chez une population vieillissante. L'intégration de médicaments antiviraux avancés et les efforts continus de R&D alimentent la croissance, le Japon privilégiant la réduction du fardeau de la grippe.
Aperçu du marché indien des médicaments contre la grippe
En 2024, le marché indien des médicaments antigrippaux représentait une part de marché significative en Asie-Pacifique, grâce à l'essor de la classe moyenne, à l'urbanisation rapide et à l'amélioration de l'accès aux soins de santé. L'Inde est un vaste marché fortement touché par les maladies infectieuses, et les médicaments antigrippaux occupent une place croissante dans les programmes de santé publique et privée. L'amélioration des infrastructures de santé et la disponibilité de médicaments antigrippaux, tant fabriqués localement qu'importés, sont des facteurs clés qui propulsent le marché indien, avec un TCAC prévu de 2,6 %.
Part de marché des médicaments contre la grippe
L’industrie des médicaments contre la grippe est principalement dirigée par des entreprises bien établies, notamment :
- AbbVie Inc. (États-Unis)
- AstraZeneca (Royaume-Uni)
- BioNTech SE (Allemagne)
- Bristol-Myers Squibb Company (États-Unis)
- Cipla (Inde)
- COCRYSTAL PHARMA, INC. (États-Unis)
- CSL (Royaume-Uni)
- Daiichi Sankyo Company, Limited (Japon)
- Dr. Reddy's Laboratories Ltd. (Inde)
- F. Hoffmann-La Roche AG (Suisse)
- Gilead Sciences, Inc. (États-Unis)
- GSK plc. (Royaume-Uni)
- Johnson & Johnson Services, Inc. (États-Unis)
- Merck & Co., Inc. (États-Unis)
- Moderna, Inc. (États-Unis)
- Novartis AG (Suisse)
- Novavax (États-Unis)
- Osivax (France)
- Sanofi SA (France)
Derniers développements sur le marché mondial des médicaments contre la grippe
- En septembre 2024, le vaccin FluMist d'AstraZeneca a été approuvé pour l'auto-administration aux États-Unis. Il s'agit d'une avancée majeure : il s'agit du premier vaccin antigrippal ne nécessitant pas l'intervention d'un professionnel de santé, améliorant ainsi son accessibilité et sa commodité pour les personnes en quête de prévention. FluMist a été approuvé par la FDA américaine pour l'auto-administration par les adultes jusqu'à 49 ans ou par un parent/tuteur pour les personnes âgées de 2 à 17 ans.
- En août 2024, des scientifiques de la faculté de médecine de Harvard ont mis au point un spray nasal appelé Spray de Capture et de Neutralisation des Pathogènes (PCANS), ou Profi. Ce spray prétend protéger contre la grippe, le rhume et la COVID-19 avec une efficacité de plus de 99,99 % en formant une barrière dans la cavité nasale qui capture et neutralise les virus et les bactéries.
- En mai 2024, Sanofi et Novavax ont annoncé un accord de licence co-exclusif pour la co-commercialisation mondiale du vaccin autonome avec adjuvant contre la COVID-19 de Novavax et le développement d'une nouvelle combinaison vaccinale grippe-COVID-19. Ce partenariat vise à accélérer le développement de vaccins combinés, offrant ainsi une plus grande commodité et une meilleure protection aux patients.
- En avril 2023, Sinovac Biotech Co., Ltd. a annoncé la mise en service récente de sa nouvelle usine de fabrication de vaccins antigrippaux à Pékin. Cette expansion accroît la capacité de production mondiale de vaccins antigrippaux, contribuant ainsi à une meilleure disponibilité et à un approvisionnement plus large.
- En mars 2023, le Comité consultatif sur les vaccins et les produits biologiques connexes (VRBPAC) de la FDA s'est réuni pour déterminer la composition du vaccin pour la saison grippale américaine 2023-2024. Ce processus annuel est essentiel pour garantir l'efficacité des vaccins contre les souches virales grippales en circulation.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.