Global Exocrine Pancreatic Insufficiency Epi Therapeutics And Diagnostics Market
Taille du marché en milliards USD
TCAC :
% 
 USD
8.81 Billion
USD
15.38 Billion
2024
2032
USD
8.81 Billion
USD
15.38 Billion
2024
2032
| 2025 –2032 | |
| USD 8.81 Billion | |
| USD 15.38 Billion | |
|
|
|
|
Segmentation du marché mondial des thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE), par diagnostic (examens d'imagerie et test de la fonction pancréatique), traitement (prise en charge nutritionnelle, thérapie de remplacement des enzymes pancréatiques [PERT]), type de médicament (générique et de marque), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile, centres de diagnostic, instituts de recherche et universitaires, etc.), canal de distribution (achat direct, pharmacies de détail, distributeurs tiers, etc.) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE)
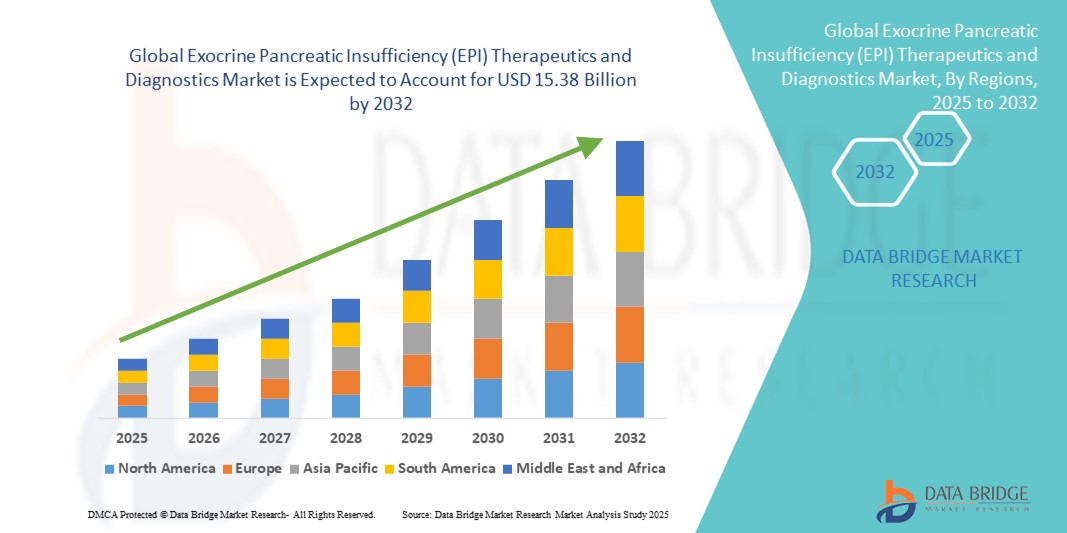
- La taille du marché mondial des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE) était évaluée à 8,81 milliards USD en 2024 et devrait atteindre 15,38 milliards USD d'ici 2032 , à un TCAC de 7,20 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par la prévalence croissante des troubles pancréatiques, la sensibilisation croissante à la santé gastro-intestinale et les progrès des technologies diagnostiques telles que les tests d'élastase fécale et les méthodes d'imagerie. Ces facteurs favorisent le dépistage précoce et la prise en charge rapide de l'insuffisance pancréatique exocrine (IPE).
- Par ailleurs, l'adoption croissante des traitements de substitution par enzymes pancréatiques (TSEP), conjuguée aux recherches continues sur de nouvelles approches thérapeutiques et à l'amélioration de la précision diagnostique, améliore significativement les résultats pour les patients. Ces facteurs convergents accélèrent l'adoption des solutions thérapeutiques et diagnostiques pour l'insuffisance pancréatique exocrine (IPE), stimulant ainsi considérablement la croissance du secteur.
Analyse du marché des thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE)
- Les thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE), comprenant des thérapies de remplacement enzymatique, des solutions de gestion nutritionnelle et des méthodes de tests diagnostiques, sont des éléments de plus en plus essentiels des soins gastro-intestinaux modernes en raison de leur capacité à améliorer l'absorption des nutriments, à améliorer les résultats des patients et à réduire les complications associées à la malnutrition.
- La demande croissante de thérapies et de diagnostics EPI est principalement alimentée par la prévalence croissante de la pancréatite chronique, de la fibrose kystique et du cancer du pancréas, associée à une sensibilisation croissante au diagnostic précoce et à une préférence croissante pour les thérapies de remplacement enzymatique comme option de traitement de première intention.
- L'Amérique du Nord a dominé le marché des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE), avec une part de chiffre d'affaires de 39,5 % en 2024. Cette région se caractérise par une infrastructure de soins de santé de pointe, une forte sensibilisation des patients et des médecins, et une forte présence d'acteurs clés du secteur. Les États-Unis ont contribué à la majeure partie du chiffre d'affaires régional, grâce à l'adoption croissante des traitements de substitution enzymatique pancréatique (TSP) et à la disponibilité d'outils diagnostiques avancés.
- L'Asie-Pacifique devrait être la région connaissant la croissance la plus rapide sur le marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE) au cours de la période de prévision, avec un TCAC projeté de 8,6 % de 2025 à 2032, grâce à la hausse des investissements dans les soins de santé, à la prévalence croissante des troubles pancréatiques, à l'amélioration de l'accès aux installations de diagnostic et à la hausse des revenus disponibles dans des pays comme la Chine et l'Inde.
- Le segment des médicaments de marque a dominé le marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE) avec une part de marché de 56,1 % en 2024, en raison de la présence établie de sociétés pharmaceutiques de premier plan, de preuves cliniques solides et d'une plus grande confiance des médecins dans les formulations PERT de marque.
Portée du rapport et segmentation du marché des thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE)
|
Attributs |
Insuffisance pancréatique exocrine (IPE) : informations clés sur le marché |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
Europe
Asie-Pacifique
Moyen-Orient et Afrique
Amérique du Sud
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
Confort amélioré grâce à des thérapies et des diagnostics avancés
- Une tendance significative et croissante sur le marché mondial des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE) est l'adoption croissante de thérapies de remplacement enzymatique avancées (PERT) et d'approches diagnostiques innovantes qui améliorent la précision du traitement, la commodité du patient et la gestion à long terme de l'IPE.
- Par exemple, les principales sociétés pharmaceutiques lancent des formulations PERT de nouvelle génération, plus stables et plus efficaces, permettant aux patients de mieux gérer la maldigestion et l'absorption des nutriments. De même, les progrès des tests diagnostiques non invasifs permettent aux cliniciens de confirmer plus facilement l'IPE à un stade plus précoce, réduisant ainsi les délais diagnostiques.
- L'intégration des données de vie réelle (RWD) et des outils d'aide à la décision clinique dans les soins prodigués par les professionnels de santé permet également d'adapter plus efficacement les schémas thérapeutiques. Ces innovations favorisent l'optimisation des dosages, minimisent les effets secondaires et garantissent une meilleure observance thérapeutique à long terme chez les patients prenant en charge des affections pancréatiques chroniques.
- De plus, les améliorations apportées à la conception des capsules et aux mécanismes d'administration des enzymes fournissent des résultats plus fiables pour les patients en garantissant que les enzymes restent actives jusqu'à ce qu'elles atteignent l'intestin grêle, améliorant ainsi l'efficacité de l'absorption et l'état nutritionnel.
- Cette tendance vers des thérapies et des diagnostics plus efficaces, plus conviviaux et technologiquement avancés transforme profondément les attentes en matière de soins liés à l'IPE. Les innovateurs pharmaceutiques se concentrent sur des thérapies à biodisponibilité accrue et des outils de diagnostic permettant d'obtenir des résultats rapides et précis.
- La demande de thérapies et de diagnostics EPI qui offrent une commodité accrue, de meilleurs résultats de traitement et une gestion complète de la maladie augmente rapidement dans les hôpitaux, les cliniques et les centres de soins spécialisés, car les patients et les prestataires accordent de plus en plus la priorité à la qualité de vie et à la santé nutritionnelle à long terme.
Dynamique du marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE)
Conducteur
Besoin croissant en raison de la sensibilisation croissante aux maladies et des thérapies avancées
- La prévalence croissante des troubles pancréatiques, associée à une sensibilisation croissante à la santé digestive et à des capacités de diagnostic améliorées, constitue un facteur important de la demande accrue de thérapies et de diagnostics pour l'insuffisance pancréatique exocrine (IPE).
- Par exemple, en avril 2024, des sociétés pharmaceutiques de premier plan ont annoncé le développement de formulations de thérapie de remplacement d'enzymes pancréatiques (TERP) de nouvelle génération visant à améliorer l'efficacité, la tolérance et l'observance thérapeutique des patients. Ces initiatives, menées par des acteurs clés, devraient stimuler la croissance du secteur des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) au cours de la période de prévision.
- Alors que les patients et les prestataires de soins de santé reconnaissent de plus en plus l’importance d’une détection précoce et d’une gestion efficace, les diagnostics avancés tels que les tests d’imagerie, les tests de la fonction pancréatique et les analyses de selles gagnent du terrain, offrant des interventions rapides et de meilleurs résultats de traitement.
- En outre, l’accent croissant mis sur la nutrition personnalisée, les thérapies combinées et les solutions de surveillance à domicile améliore les soins centrés sur le patient dans les contextes cliniques et ambulatoires, favorisant une adoption plus large des thérapies et des diagnostics EPI.
- La commodité des enzymothérapies orales, la prise en charge nutritionnelle ciblée et l'accessibilité des services de diagnostic sont des facteurs clés qui favorisent l'adoption des solutions pour l'insuffisance pancréatique exocrine (IPE) dans les hôpitaux, les cliniques spécialisées et les services de soins à domicile. La disponibilité croissante de protocoles de traitement conviviaux et standardisés contribue également à la croissance du marché.
Retenue/Défi
Coûts de traitement élevés et sensibilisation limitée dans les marchés émergents
- Le coût relativement élevé des formulations PERT avancées et des tests diagnostiques spécialisés constitue un obstacle majeur à une pénétration plus large du marché. Dans les régions sensibles aux prix, l'accès limité aux infrastructures de santé et les contraintes financières peuvent freiner l'adoption de ces technologies.
- De plus, le manque de sensibilisation aux symptômes de l’EPI et le sous-diagnostic dans certaines populations ont rendu la détection et le traitement précoces difficiles, ce qui peut retarder le début du traitement.
- Il est essentiel de relever ces défis par le biais de campagnes d’éducation des patients, d’une extension de la couverture d’assurance et du développement de diagnostics et de thérapies rentables pour élargir l’accès au marché.
- En outre, la recherche et l’innovation continues visant à produire des formulations de remplacement enzymatique abordables et efficaces seront essentielles pour soutenir la croissance du marché à l’échelle mondiale.
- Bien que les prix de certaines thérapies enzymatiques génériques diminuent progressivement, la prime perçue pour les solutions diagnostiques de marque ou avancées peut encore freiner leur adoption, notamment dans les régions en développement. Relever ces défis grâce à une meilleure sensibilisation des professionnels de santé, une optimisation des coûts et des recommandations thérapeutiques standardisées sera essentiel à l'expansion à long terme du secteur.
Portée du marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE)
Le marché est segmenté en fonction du diagnostic, du traitement, du type de médicament, de l’utilisateur final et du canal de distribution.
- Par diagnostic
Sur la base du diagnostic, le marché des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) est segmenté en deux catégories : les tests d'imagerie et les tests de la fonction pancréatique. En 2024, le segment des tests de la fonction pancréatique a dominé la plus grande part de chiffre d'affaires du marché, avec 52,3 %, grâce à sa grande précision dans l'évaluation du déficit enzymatique, sa large adoption en pratique clinique et son rôle crucial dans l'orientation des plans de traitement des patients atteints de pancréatite chronique et de mucoviscidose. Les tests de la fonction pancréatique sont considérés comme essentiels pour la détection précoce et la surveillance continue, offrant des informations exploitables aux cliniciens comme aux patients. Ils fournissent des résultats fiables pour l'ajustement du traitement et la prise en charge à long terme. Ce segment bénéficie également d'une couverture de remboursement croissante sur les principaux marchés développés. De plus, les professionnels de santé privilégient les tests de la fonction pancréatique pour leur précision diagnostique et leur capacité à compléter d'autres évaluations cliniques.
Le segment des examens d'imagerie devrait connaître le TCAC le plus rapide, soit 7,9 % entre 2025 et 2032, grâce aux progrès des technologies d'imagerie non invasives telles que l'IRM et la tomodensitométrie, à la sensibilisation accrue au diagnostic précoce et à leur adoption croissante dans les cliniques spécialisées et les centres de diagnostic. Les examens d'imagerie fournissent des informations complémentaires aux analyses fonctionnelles, contribuant ainsi à une évaluation complète de la morphologie pancréatique et des complications associées. Le développement des infrastructures de santé en Asie-Pacifique et en Amérique latine favorise une adoption rapide. Les innovations technologiques ont amélioré la résolution de l'imagerie, facilitant la détection de modifications pancréatiques subtiles. La réduction des coûts des équipements d'imagerie stimule également leur adoption. Globalement, les examens d'imagerie deviennent un choix privilégié pour les cliniciens à la recherche d'un profil diagnostique complet.
- Par traitement
En termes de traitement, le marché des thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE) est segmenté en deux catégories : la prise en charge nutritionnelle et le traitement substitutif par enzymes pancréatiques (TSEP). En 2024, le segment des TEP a dominé la plus grande part de marché, avec 48,7 % de chiffre d'affaires, grâce à son efficacité prouvée pour améliorer l'absorption des nutriments, réduire la malnutrition et améliorer la qualité de vie des patients atteints d'IPE. Largement recommandée par les gastro-entérologues, la TEP bénéficie d'une forte préférence des médecins et des patients en Amérique du Nord et en Europe. Elle garantit une administration standardisée des enzymes et des résultats thérapeutiques constants. Son adoption est soutenue par des recommandations cliniques exhaustives. Ce segment bénéficie d'innovations produits continues améliorant la stabilité et la biodisponibilité des enzymes. Les programmes d'observance thérapeutique des patients renforcent également la position des thérapies TEP sur le marché.
Le segment de la gestion nutritionnelle devrait connaître le TCAC le plus rapide, soit 8,4 % entre 2025 et 2032, grâce à l'adoption croissante de régimes alimentaires spécialisés, de compléments nutritionnels riches en protéines et de plans nutritionnels personnalisés qui soutiennent l'enzymothérapie, en particulier dans les marchés émergents où les stratégies de gestion de l'IPE sont de plus en plus connues. La gestion nutritionnelle complète le traitement PERT en s'attaquant à la malnutrition globale et en soutenant la santé des patients. L'importance croissante accordée aux interventions sur le mode de vie et aux programmes dirigés par des diététiciens stimule la croissance. La disponibilité de formulations nutritionnelles prêtes à l'emploi accélère l'observance thérapeutique des patients. Le développement des services de soins à domicile pour le soutien nutritionnel renforce l'adoption de ces solutions par le marché. Les marchés émergents investissent de plus en plus dans des programmes d'éducation des patients mettant l'accent sur la gestion nutritionnelle.
- Par type de médicament
Selon le type de médicament, le marché des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) est segmenté en génériques et en médicaments de marque. En 2024, le segment des médicaments de marque a dominé la plus grande part de marché, avec 56,1 %, grâce à la présence établie de laboratoires pharmaceutiques de premier plan, à des preuves cliniques solides et à la confiance accrue des médecins dans les formulations PERT de marque. Les médicaments de marque offrent souvent une meilleure stabilité, une teneur en enzymes standardisée et des autorisations réglementaires qui favorisent une adoption généralisée. Ils bénéficient de campagnes marketing et de sensibilisation efficaces. Ce segment est soutenu par des réseaux de distribution exclusifs sur les marchés développés. Les médicaments de marque conservent la confiance des patients grâce à une qualité constante. Les essais cliniques confirment l'efficacité des formulations de marque.
Le segment des médicaments génériques devrait connaître le TCAC le plus rapide, soit 9,2 %, entre 2025 et 2032, grâce à la rentabilité, à l'augmentation de la couverture santé et à l'élargissement de la disponibilité dans les régions en développement. Les formulations génériques rendent les traitements EPI plus accessibles à un plus large public. L'augmentation des autorisations réglementaires pour les génériques encourage les fabricants à entrer sur le marché. L'acceptation des patients et des professionnels de santé progresse grâce à une efficacité comparable à celle des médicaments de marque. L'approvisionnement en gros par les hôpitaux et les pharmacies d'officine soutient la croissance. L'accessibilité financière favorise l'adoption sur les marchés émergents. Les programmes de formation des cliniciens sur l'efficacité des génériques contribuent également à l'adoption de ces médicaments.
- Par utilisateur final
En fonction de l'utilisateur final, le marché des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) est segmenté en hôpitaux, cliniques spécialisées, soins à domicile, centres de diagnostic, instituts de recherche et universitaires, entre autres. Le segment hospitalier a dominé la plus grande part de marché, avec 50,4 % de chiffre d'affaires en 2024, grâce à une forte fréquentation, des établissements de soins intégrés et de solides réseaux de médecins gérant les traitements et diagnostics de l'IPE. Les hôpitaux servent de centres principaux pour le diagnostic et l'administration du PERT, garantissant l'observance et le suivi continu. Ils offrent une prise en charge complète, incluant des conseils diététiques et des services de suivi. Les circuits de distribution et d'approvisionnement établis dans les hôpitaux renforcent les parts de marché. Les hôpitaux pilotent également les essais cliniques et les collaborations de recherche pour la prise en charge de l'IPE. La préférence institutionnelle pour les thérapies standardisées renforce la domination du marché.
Le segment des soins à domicile devrait connaître le TCAC le plus rapide, soit 10,1 % entre 2025 et 2032, grâce à la préférence croissante des patients pour l'enzymothérapie à domicile, la télésurveillance et l'adoption croissante des plateformes de télémédecine pour la prise en charge des maladies. Les soins à domicile améliorent le confort et l'observance thérapeutique des patients. Le développement du recours à des prestataires spécialisés en soins à domicile facilite la mise en œuvre des traitements. L'éducation et la télésurveillance à distance améliorent l'efficacité des traitements. La croissance du segment est accélérée par une couverture d'assurance maladie favorable aux soins à domicile. Les campagnes de sensibilisation ciblant les patients et les soignants encouragent la prise en charge à domicile. La mise en place de PERT et d'un soutien nutritionnel par la technologie stimule l'adoption de ces solutions.
- Par canal de distribution
En fonction du canal de distribution, le marché des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) est segmenté en appels d'offres directs, pharmacies de détail, distributeurs tiers et autres. Le segment des appels d'offres directs a dominé la plus grande part de chiffre d'affaires du marché, avec 45,6 % en 2024, grâce aux achats en gros effectués par les hôpitaux, les cliniques spécialisées et les programmes gouvernementaux, garantissant un approvisionnement constant et des avantages en termes de coûts pour les gros acheteurs. Les accords d'appels d'offres directs incluent souvent des prix et des contrats d'approvisionnement préférentiels. Les hôpitaux et les grandes institutions privilégient ce canal pour sa fiabilité. Des réseaux logistiques solides favorisent l'efficacité de la distribution. Ce segment garantit l'accès à des formulations de marque à grande échelle. Les initiatives gouvernementales en matière de sensibilisation à l'IPE et de couverture thérapeutique renforcent encore ce canal. Les contrats à long terme préservent la stabilité du marché et réduisent les ruptures de stock.
Le secteur des pharmacies de détail devrait connaître le TCAC le plus rapide, soit 9,5 % entre 2025 et 2032, grâce à une sensibilisation croissante des patients, à la disponibilité sans ordonnance de compléments nutritionnels et de produits PERT, et au développement des réseaux de distribution en zones urbaines et semi-urbaines. Les pharmacies de détail offrent aux patients un accès pratique et immédiat aux traitements. Une meilleure éducation des patients à la gestion des EPI favorise l'adoption de ces traitements. Les partenariats avec les fabricants améliorent la disponibilité des stocks. L'expansion de la distribution sur les marchés émergents améliore l'accessibilité. Ce secteur bénéficie de campagnes marketing ciblant les soignants et les patients. La préférence croissante pour l'auto-administration des traitements à domicile stimule la croissance des circuits de distribution.
Analyse régionale du marché des thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE)
- L'Amérique du Nord a dominé le marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE) avec la plus grande part de revenus de 39,5 % en 2024, caractérisée par une infrastructure de soins de santé avancée, des niveaux élevés de sensibilisation parmi les patients et les médecins et la forte présence d'acteurs clés de l'industrie.
- La croissance du marché est tirée par l’adoption croissante des thérapies de remplacement des enzymes pancréatiques (PERT), l’amélioration des solutions de gestion nutritionnelle et la disponibilité d’outils de diagnostic avancés pour la détection précoce et la surveillance de l’insuffisance pancréatique.
- Des activités de recherche robustes, une attention croissante portée aux troubles digestifs liés au corps et une couverture sanitaire étendue soutiennent davantage l'expansion du marché dans la région.
Analyse du marché américain des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
Le marché américain des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) a représenté la majeure partie du chiffre d'affaires nord-américain en 2024, grâce à une forte sensibilisation des patients, à des recherches cliniques approfondies et à l'adoption rapide d'options thérapeutiques innovantes. La prévalence croissante des troubles pancréatiques chroniques, conjuguée à la disponibilité de plateformes diagnostiques complètes telles que l'imagerie et les tests de la fonction pancréatique, stimule une demande importante. De plus, le développement de nouvelles formulations PERT avancées et l'accent mis sur les approches thérapeutiques centrées sur le patient stimulent la croissance du marché.
Analyse du marché européen des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
Le marché européen des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) devrait connaître une croissance significative au cours de la période de prévision, principalement portée par la prévalence croissante des troubles pancréatiques, la hausse des dépenses de santé et un cadre réglementaire favorable aux diagnostics et traitements. La sensibilisation croissante des professionnels de santé au diagnostic précoce et aux stratégies thérapeutiques optimales favorise l'adoption d'approches pharmacologiques et nutritionnelles. Les principaux pays européens réalisent des investissements importants dans les infrastructures de santé, améliorant ainsi l'accès des patients atteints d'IPE à des cliniques spécialisées et à des installations de diagnostic.
Analyse du marché britannique des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
Le marché britannique des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) devrait connaître une croissance significative au cours de la période de prévision, portée par une sensibilisation accrue à la santé digestive, l'augmentation des taux de diagnostic et l'adoption du PERT et d'une prise en charge nutritionnelle de soutien. De plus, le développement des cliniques spécialisées et des centres de recherche sur les troubles pancréatiques contribue au développement du marché, tandis que les programmes de santé favorisant une intervention précoce encouragent une adoption plus large des traitements.
Analyse du marché allemand des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
Le marché allemand des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) devrait connaître une croissance annuelle moyenne (TCAC) considérable, portée par l'importance accordée aux soins de santé de précision, les investissements croissants dans les technologies diagnostiques et la demande de solutions thérapeutiques de pointe. La prévalence croissante de l'insuffisance pancréatique, conjuguée à des infrastructures hospitalières performantes et à un niveau élevé d'éducation des patients, favorise l'adoption de l'enzymothérapie substitutive et des tests diagnostiques avancés.
Analyse du marché des thérapies et diagnostics de l'insuffisance pancréatique exocrine (IPE) en Asie-Pacifique
Le marché des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE) en Asie-Pacifique devrait connaître la croissance la plus rapide au cours de la période de prévision, avec un TCAC prévu de 8,6 % entre 2025 et 2032. Cette croissance est tirée par la hausse des investissements dans les soins de santé, la prévalence croissante des troubles pancréatiques, l'amélioration de l'accès aux installations de diagnostic et la hausse des revenus disponibles dans des pays comme la Chine et l'Inde. L'intensification des campagnes de sensibilisation à la santé publique, les initiatives gouvernementales en faveur de la santé digestive et le développement des capacités locales de production pharmaceutique accélèrent la pénétration du marché.
Analyse du marché japonais des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
Le marché japonais des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) connaît un essor important en raison de l'urbanisation rapide, du vieillissement de la population et de l'attention croissante portée à la santé digestive et pancréatique. L'adoption de thérapies PERT avancées et de solutions diagnostiques complètes est soutenue par un système de santé performant sur le plan technologique et une sensibilisation croissante des patients.
Analyse du marché chinois des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
En 2024, le marché chinois des traitements et diagnostics de l'insuffisance pancréatique exocrine (IPE) a représenté une part significative du chiffre d'affaires en Asie-Pacifique, grâce au développement des infrastructures de santé, à la croissance de la classe moyenne et aux initiatives gouvernementales visant à améliorer le diagnostic et le traitement précoces des troubles pancréatiques. La prévalence croissante des troubles digestifs et l'élargissement de l'accès aux soins en zones urbaines et semi-urbaines sont des facteurs clés de la croissance du marché.
Part de marché des thérapies et des diagnostics de l'insuffisance pancréatique exocrine (IPE)
L'industrie thérapeutique et diagnostique de l'insuffisance pancréatique exocrine (IPE) est principalement dirigée par des entreprises bien établies, notamment :
- EagleBio (États-Unis)
- AbbVie Inc. (États-Unis)
- Nordmark Pharma GmbH. (Allemagne)
- Digestive Care, Inc. (États-Unis)
- LUMITOS AG (Allemagne)
- Alcresta Therapeutics, Inc. (États-Unis)
- ChiRhoClin, Inc. (États-Unis)
- Abbott (États-Unis)
- Bioserv Analytics and Medical Devices GmbH (Allemagne)
- LabCorp (États-Unis)
- Groupe de sociétés Organon (États-Unis)
- Metagenics LLC (États-Unis)
- Johnson & Johnson et ses filiales (États-Unis)
- Nestlé Health Science (Suisse)
- VIVUS LLC (États-Unis)
- ScheBo Biotech AG (Allemagne)
Développements récents sur le marché mondial des thérapies et diagnostics pour l'insuffisance pancréatique exocrine (IPE)
- En mai 2025, une étude publiée dans le Pharmaceutical Journal a souligné que l'insuffisance pancréatique exocrine (IPE) survient lorsque le pancréas est incapable de produire suffisamment d'enzymes pour faciliter la digestion, ce qui entraîne une malnutrition due à une absorption inadéquate des nutriments.
- En août 2021, AzurRx a annoncé qu'elle s'était engagée dans le développement d'une lipase dérivée de levure, MS1819, qui a été conçue pour avoir une activité enzymatique supérieure par rapport aux traitements actuels
- En février 2023, Codexis, Inc. et Nestlé Health Science ont annoncé les résultats intermédiaires d'un essai de phase I examinant l'innocuité, la tolérance, la pharmacocinétique (PK) et la pharmacodynamie du CDX-7108. Une variante de lipase appelée CDX-7108 a été créée expressément pour pallier les inconvénients du traitement substitutif enzymatique pancréatique (TREP) actuel. Cela a facilité la commercialisation du produit.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.