Global Denys Drash Syndrome Market
Taille du marché en milliards USD
TCAC :
% 
 USD
343.72 Million
USD
547.84 Million
2024
2032
USD
343.72 Million
USD
547.84 Million
2024
2032
| 2025 –2032 | |
| USD 343.72 Million | |
| USD 547.84 Million | |
|
|
|
|
Segmentation du marché mondial du syndrome de Denys-Drash, par diagnostic (analyses de laboratoire, imagerie, biopsie rénale, etc.), traitement (thérapie de remplacement rénal, transplantation rénale, médicaments, etc.), âge d'apparition (enfance, adolescence, nourrisson et nouveau-né), voie d'administration (orale, parentérale, etc.), utilisateurs finaux (hôpitaux, cliniques spécialisées, soins à domicile, etc.), canal de distribution (pharmacie hospitalière, pharmacie de détail, pharmacie en ligne, etc.) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché du syndrome de Denys-Drash
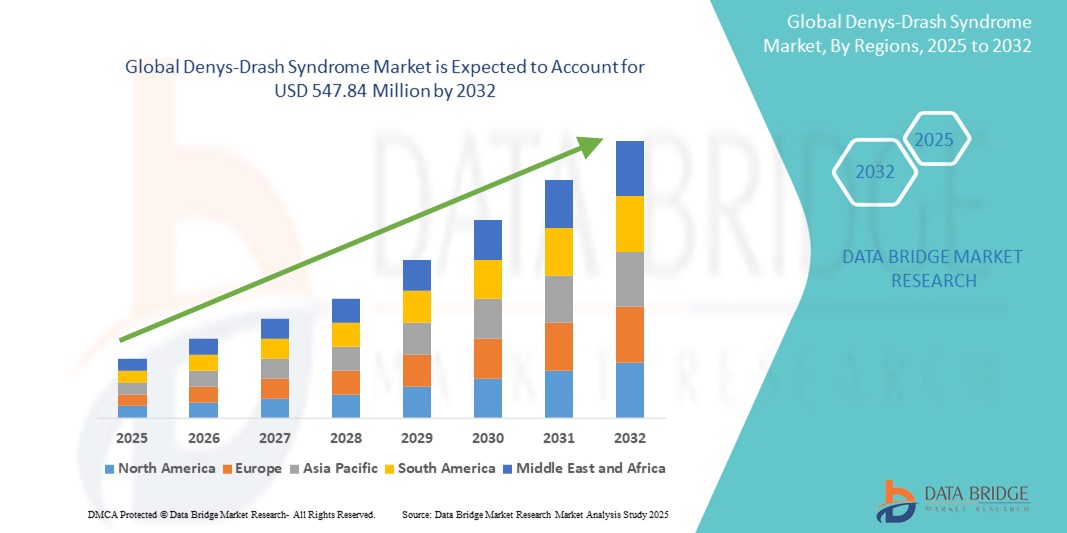
- La taille du marché mondial du syndrome de Denys-Drash était évaluée à 343,72 millions USD en 2024 et devrait atteindre 547,84 millions USD d'ici 2032 , à un TCAC de 6,00 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par une sensibilisation accrue, un diagnostic génétique précoce et les progrès de la médecine moléculaire et de la néphrologie, qui améliorent considérablement la prise en charge des maladies génétiques rares telles que le syndrome de Denys-Drash (SDD). L'amélioration des capacités de dépistage prénatal et postnatal a contribué à une identification précoce et à une meilleure évaluation pronostique du SDD.
- De plus, la demande croissante d'interventions thérapeutiques efficaces, telles que les traitements néphroprotecteurs, les traitements hormonaux substitutifs et les interventions chirurgicales, fait des modèles de soins complets la norme pour la prise en charge du syndrome de Denys-Drash. Ces facteurs convergents accélèrent l'adoption de solutions ciblées pour le syndrome de Denys-Drash, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché du syndrome de Denys-Drash
- Le syndrome de Denys-Drash (SDD), une maladie génétique rare caractérisée par une triade de néphropathie, de pseudohermaphrodisme masculin et de prédisposition à la tumeur de Wilms, fait l'objet d'une recherche et d'une sensibilisation accrues, ce qui contribue à de meilleures approches diagnostiques et thérapeutiques dans les systèmes de santé.
- La demande croissante d’outils de diagnostic améliorés et de thérapies d’intervention précoce est principalement motivée par une sensibilisation croissante aux maladies rares pédiatriques, aux progrès des tests génétiques et à l’augmentation du financement gouvernemental et privé dans la recherche sur les maladies orphelines.
- L'Amérique du Nord a dominé le marché du syndrome de Denys-Drash, avec une part de chiffre d'affaires de 41,6 % en 2024. Cette croissance est due à une infrastructure de santé de pointe, à l'adoption précoce des diagnostics génétiques et à la forte présence de grandes entreprises pharmaceutiques et biotechnologiques spécialisées dans le traitement des maladies rares. Les États-Unis, en particulier, connaissent une croissance significative grâce à des initiatives comme le Réseau de recherche clinique sur les maladies rares (RDCRN) et à un nombre croissant d'essais cliniques axés sur les néphropathies pédiatriques et la tumeur de Wilms.
- L'Asie-Pacifique devrait connaître le TCAC le plus rapide, soit 9,7 %, au cours de la période de prévision, grâce à l'amélioration de l'accès aux soins de santé, à l'augmentation des investissements dans la recherche sur les maladies rares et au développement des programmes de sensibilisation dans des pays comme la Chine, l'Inde et le Japon.
- Le segment des tests de laboratoire a dominé le marché du syndrome de Denys-Drash, avec une part de marché de 39,6 % en 2024, grâce à une dépendance accrue aux tests génétiques et biochimiques, en particulier à l'analyse des mutations du gène WT1, pour un diagnostic précoce.
Portée du rapport et segmentation du marché du syndrome de Denys-Drash
|
Attributs |
Syndrome de Denys-Drash : principales perspectives du marché |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
Europe
Asie-Pacifique
Moyen-Orient et Afrique
Amérique du Sud
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché du syndrome de Denys-Drash
« Accent accru sur la détection précoce et le traitement personnalisé »
- Une tendance significative et croissante sur le marché mondial du syndrome de Denys-Drash est l'importance croissante accordée au diagnostic précoce et à la mise en œuvre de thérapies ciblées. Le syndrome de Denys-Drash étant une maladie génétique rare et grave, son identification précoce grâce à un dépistage génétique avancé et à des tests prénataux est devenue essentielle pour améliorer les résultats des patients.
- Par exemple, les tests de laboratoire, notamment le séquençage du gène WT1, sont de plus en plus utilisés pour les cas suspects chez les nourrissons présentant des anomalies génitales et un syndrome néphrotique précoce. Ces avancées en matière de diagnostic moléculaire facilitent un diagnostic précoce et une intervention clinique rapide.
- L'essor de la médecine de précision façonne également les stratégies thérapeutiques sur le marché du syndrome de Denys-Drash. Les médecins utilisent désormais des plans de traitement personnalisés, basés sur les corrélations génotype-phénotype, pour gérer les symptômes et améliorer les taux de survie.
- La transplantation rénale demeure un pilier essentiel du traitement des patients évoluant vers une insuffisance rénale terminale, avec de meilleurs résultats chirurgicaux et une meilleure survie du greffon, augmentant ainsi l'espérance de vie. De plus, les traitements hormonaux substitutifs sont adaptés aux profils hormonaux individuels des patients atteints de dysgénésie gonadique.
- Par ailleurs, les organisations de santé mondiales et les réseaux de lutte contre les maladies rares collaborent pour sensibiliser davantage, simplifier l'orientation des patients et fournir des ressources aux familles touchées, notamment dans les régions mal desservies. Par exemple, la base de données Orphanet et le Réseau de recherche clinique sur les maladies rares (RDCRN) proposent des registres détaillés qui facilitent la recherche clinique et le développement thérapeutique pour le syndrome de Denys-Drash.
- La disponibilité croissante des soins de soutien, notamment la dialyse à domicile et l'administration d'hormonothérapie, contribue à une meilleure qualité de vie des patients, tout en élargissant le rôle des soins à domicile dans la gestion des maladies rares.
Dynamique du marché du syndrome de Denys-Drash
Conducteur
« Besoin croissant de diagnostic précoce et de prise en charge des maladies rares »
- La sensibilisation croissante aux maladies génétiques rares, associée aux progrès du dépistage génétique et des diagnostics pédiatriques, stimule considérablement la demande d'identification et de traitement précoces du syndrome de Denys-Drash (SDD).
- Par exemple, en mai 2024, Sanofi a annoncé l'expansion de son programme de diagnostic des maladies rares, visant à améliorer la détection précoce des syndromes congénitaux comme le syndrome de Denys-Drash grâce à un accès élargi au séquençage de nouvelle génération (NGS). De telles initiatives devraient stimuler la croissance du marché du syndrome de Denys-Drash au cours de la période de prévision.
- À mesure que les professionnels de santé et les soignants sont mieux informés sur les symptômes et les complications à long terme associés au syndrome de Down (SD) – telles que la tumeur de Wilms, la néphropathie et la dysgénésie gonadique –, l'accent est mis sur les stratégies d'intervention précoce. Celles-ci incluent la néphrectomie, l'hormonothérapie et le conseil génétique pour améliorer la qualité de vie des patients.
- De plus, l'essor des soins rénaux pédiatriques et des programmes de traitement multidisciplinaires dans les hôpitaux et les cliniques spécialisées améliore l'accès à des soins complets pour les enfants atteints. Ces avancées favorisent un diagnostic précoce, réduisent les risques de mortalité et améliorent les résultats pour les patients.
- L'augmentation des investissements dans la recherche sur les maladies rares, les incitations réglementaires pour les médicaments orphelins et les campagnes de sensibilisation et de défense des patients contribuent également à l'expansion du marché. Le soutien gouvernemental et les initiatives à but non lucratif accélèrent les efforts de diagnostic, notamment en Amérique du Nord et en Europe.
Retenue/Défi
« Disponibilité limitée des traitements et défis diagnostiques »
- La rareté du syndrome de Denys-Drash pose d'importants défis diagnostiques et thérapeutiques, entraînant de fréquents diagnostics erronés ou des retards de prise en charge. Ses caractéristiques cliniques, similaires à celles d'autres syndromes néphrotiques, rendent son identification précoce difficile, notamment dans les contextes à faibles ressources.
- Par exemple, l’absence de protocoles de dépistage standardisés dans les systèmes de santé ruraux entraîne souvent une détection précoce manquée du DDS, ce qui retarde les interventions appropriées et augmente le risque de complications telles que l’insuffisance rénale ou les tumeurs malignes.
- De plus, l'absence de traitements spécifiques à la maladie demeure une lacune critique. La prise en charge est souvent symptomatique, impliquant la chirurgie ou la dialyse, sans options pharmacologiques curatives. Cela limite le pipeline thérapeutique et décourage les investissements pharmaceutiques dans un petit bassin de patients.
- Les coûts élevés associés aux tests génétiques, aux soins néphrologiques pédiatriques et au suivi continu constituent également des obstacles, notamment dans les pays en développement. La couverture des maladies rares par l'assurance maladie reste inégale selon les régions, ce qui complique encore davantage l'accès aux traitements.
- Pour relever ces défis, il faut élargir l’accès au séquençage de nouvelle génération, former les médecins à la reconnaissance des maladies rares, établir des partenariats public-privé pour développer des thérapies ciblées et renforcer les politiques de santé pour soutenir les infrastructures de lutte contre les maladies rares à l’échelle mondiale.
Portée du marché du syndrome de Denys-Drash
Le marché est segmenté en fonction du diagnostic, du traitement, de l’âge d’apparition, de la voie d’administration, des utilisateurs finaux et du canal de distribution.
- Par diagnostic
Sur la base du diagnostic, le marché du syndrome de Denys-Drash est segmenté en tests de laboratoire, examens d'imagerie, biopsies rénales, etc. En 2024, le segment des tests de laboratoire représentait la plus grande part de chiffre d'affaires du marché, soit 39,6 %, grâce au recours accru aux analyses génétiques et biochimiques, notamment l'analyse des mutations du gène WT1, pour un diagnostic précoce.
Le segment des tests d'imagerie devrait connaître le TCAC le plus rapide de 21,3 % entre 2025 et 2032, alimenté par les progrès des techniques d'échographie prénatale et néonatale pour identifier les anomalies rénales et les anomalies urogénitales associées.
- Par traitement
En fonction du traitement, le marché est segmenté en thérapie de suppléance rénale, transplantation rénale, médicaments et autres. Le segment de la thérapie de suppléance rénale détenait la plus grande part de marché, soit 37,4 % en 2024, en raison de son rôle essentiel dans la prise en charge de l'insuffisance rénale terminale chez les personnes atteintes.
Le segment de la transplantation rénale devrait connaître le TCAC le plus rapide de 22,8 % au cours de la période de prévision, grâce à l'amélioration des résultats de transplantation et à la disponibilité croissante des centres de soins de néphrologie pédiatrique à l'échelle mondiale.
- Par âge d'apparition
En fonction de l'âge d'apparition, le marché est segmenté en enfance, adolescence, petite enfance et néonatologie. Le segment néonatal a dominé le marché en 2024 avec une part de chiffre d'affaires de 42,7 %, car des symptômes tels que le syndrome néphrotique et l'ambiguïté génitale se manifestent généralement dès les premières semaines de vie.
Le segment de l’enfance devrait connaître une croissance annuelle composée (TCAC) la plus rapide, soit 19,6 %, entre 2025 et 2032, reflétant les cas de diagnostic tardif ou la progression des symptômes après la naissance.
- Par voie d'administration
En fonction de la voie d'administration, le marché est segmenté en deux catégories : voie orale, voie parentérale et autres. Le segment oral a représenté la plus grande part de marché, soit 45,1 % en 2024, car les traitements hormonaux substitutifs et les médicaments de soutien sont fréquemment administrés par voie orale.
Le segment parentéral devrait croître au TCAC le plus élevé de 20,2 %, soutenu par les thérapies intraveineuses telles que les immunosuppresseurs et la nutrition parentérale utilisées lors de complications rénales.
- Par les utilisateurs finaux
En fonction des utilisateurs finaux, le marché du syndrome de Denys-Drash est segmenté en hôpitaux, cliniques spécialisées, soins à domicile et autres. Le segment hospitalier représentait la plus grande part de marché, soit 48,6 % en 2024, grâce à la disponibilité d'installations de diagnostic avancées, de soins multidisciplinaires et d'unités de transplantation rénale.
Le segment des soins à domicile devrait connaître le TCAC le plus rapide de 23,1 % au cours de la période de prévision, grâce à l'adoption croissante de la dialyse à domicile et de l'administration de médicaments pour les soins de longue durée.
- Par canal de distribution
En fonction du canal de distribution, le marché est segmenté en pharmacies hospitalières, pharmacies de détail, pharmacies en ligne, etc. En 2024, la pharmacie hospitalière a dominé le marché avec une part de marché de 40,9 %, grâce à l'accès direct aux médicaments et traitements prescrits après le diagnostic.
Le segment des pharmacies en ligne devrait connaître une croissance au TCAC le plus élevé de 25,6 %, soutenu par la pénétration croissante du commerce électronique, la commodité et la livraison à domicile de médicaments contre les maladies rares et de thérapies hormonales.
Analyse régionale du marché du syndrome de Denys-Drash
- L'Amérique du Nord a dominé le marché du syndrome de Denys-Drash avec la plus grande part de revenus de 41,6 % en 2024, grâce à une sensibilisation accrue aux maladies rénales pédiatriques rares, à des taux plus élevés de tests génétiques et à une infrastructure de diagnostic précoce dans toute la région.
- Des systèmes de remboursement des soins de santé favorables, une prévalence croissante de la tumeur de Wilms et de la néphropathie, ainsi qu'une forte présence de grandes sociétés pharmaceutiques et biotechnologiques spécialisées dans les maladies orphelines renforcent encore la domination du marché.
- La région devrait connaître une croissance de 8,3 % entre 2025 et 2032 en raison des progrès continus de la génomique et des interventions thérapeutiques.
Aperçu du marché américain du syndrome de Denys-Drash
Le marché américain du syndrome de Denys-Drash a représenté la plus grande part de chiffre d'affaires en Amérique du Nord en 2024, avec 81,2 %, grâce à une infrastructure de néphrologie pédiatrique robuste et à l'accès aux tests génomiques. Un financement fédéral important, des essais cliniques et une sensibilisation aux maladies rares favorisent encore davantage l'adoption du traitement. Le marché américain devrait croître à un TCAC de 8,7 % au cours de la période de prévision.
Aperçu du marché européen du syndrome de Denys-Drash
Le marché européen du syndrome de Denys-Drash détenait une part de revenus de 30,4 % en 2024. Cette croissance est tirée par les plans de lutte contre les maladies rares soutenus par le gouvernement, la précision croissante des diagnostics et l'intégration croissante des registres de patients dans des pays comme l'Allemagne, la France et le Royaume-Uni. L'augmentation du financement de la recherche transfrontalière par la Commission européenne y contribue également de manière significative.
Aperçu du marché du syndrome de Denys-Drash au Royaume-Uni
Le marché britannique du syndrome de Denys-Drash représentait 24,6 % de la part de marché européenne en 2024. Les initiatives croissantes de dépistage génomique du NHS, ainsi que l'adoption de diagnostics basés sur l'IA et de soins précoces pour les nourrissons, soutiennent la croissance du marché.
Aperçu du marché allemand du syndrome de Denys-Drash
En 2024, le marché allemand du syndrome de Denys-Drash représentait 21,9 % du marché européen. La solide base de recherche universitaire du pays en néphrologie et ses investissements dans les diagnostics de séquençage de nouvelle génération offrent d'importantes opportunités de croissance. L'accès des patients à un diagnostic précoce et leur participation à des essais internationaux sur les maladies rares renforcent cette dynamique.
Aperçu du marché du syndrome de Denys-Drash en Asie-Pacifique
Le marché du syndrome de Denys-Drash en Asie-Pacifique devrait connaître sa plus forte croissance (TCAC) entre 2025 et 2032, avec un taux de croissance annuel composé (TCAC) de 9,7 %, porté par l'urbanisation croissante, la hausse des revenus disponibles et la sensibilisation accrue aux maladies rares. La région représentait 19,7 % du marché en 2024. Les initiatives gouvernementales en faveur du dépistage génétique néonatal et le développement des infrastructures de santé dans des pays comme la Chine, le Japon et l'Inde constituent des moteurs de croissance majeurs.
Aperçu du marché japonais du syndrome de Denys-Drash
En 2024, le marché japonais du syndrome de Denys-Drash représentait 28,5 % du marché Asie-Pacifique et devrait croître à un TCAC de 10,8 %. L'intégration de la médecine génomique dans les soins de santé nationaux, ainsi que l'augmentation des investissements publics et privés dans la prise en charge des maladies rares pédiatriques, accélèrent l'expansion du marché.
Aperçu du marché chinois du syndrome de Denys-Drash
Le marché chinois du syndrome de Denys-Drash a conquis la plus grande part de marché en Asie-Pacifique, avec 38,9 % en 2024, grâce à une urbanisation rapide, des taux de natalité élevés et l'expansion des programmes de diagnostic précoce. Grâce au soutien des politiques nationales et à la croissance significative du secteur des biotechnologies, le marché chinois devrait connaître un TCAC de 11,9 % d'ici 2032.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.