Marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon, par cause (maladie à coronavirus 2019 (COVID-19), septicémie, inhalation de substances nocives, pneumonie sévère et autres), type (diagnostic et traitement), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile et autres), canal de distribution (appel d'offres direct, pharmacie hospitalière, pharmacie de détail et pharmacie en ligne) - Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et perspectives du marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon
La prévalence croissante des maladies infectieuses et respiratoires telles que la COVID-19 et le syndrome de détresse respiratoire aiguë, ainsi que l'attention portée au développement de vaccins et de produits thérapeutiques et diagnostiques pour ces pathologies ont renforcé la demande du marché. Les progrès technologiques pour un approvisionnement facile des produits et des installations de fabrication rapides contribuent également à la croissance du marché. Les principaux acteurs du marché se concentrent fortement sur les lancements et les approbations de produits pendant cette période cruciale. En outre, le gouvernement et les organismes de réglementation soutiennent les acteurs du marché dans l'approbation des produits en raison de l'émergence croissante de nouveaux produits.
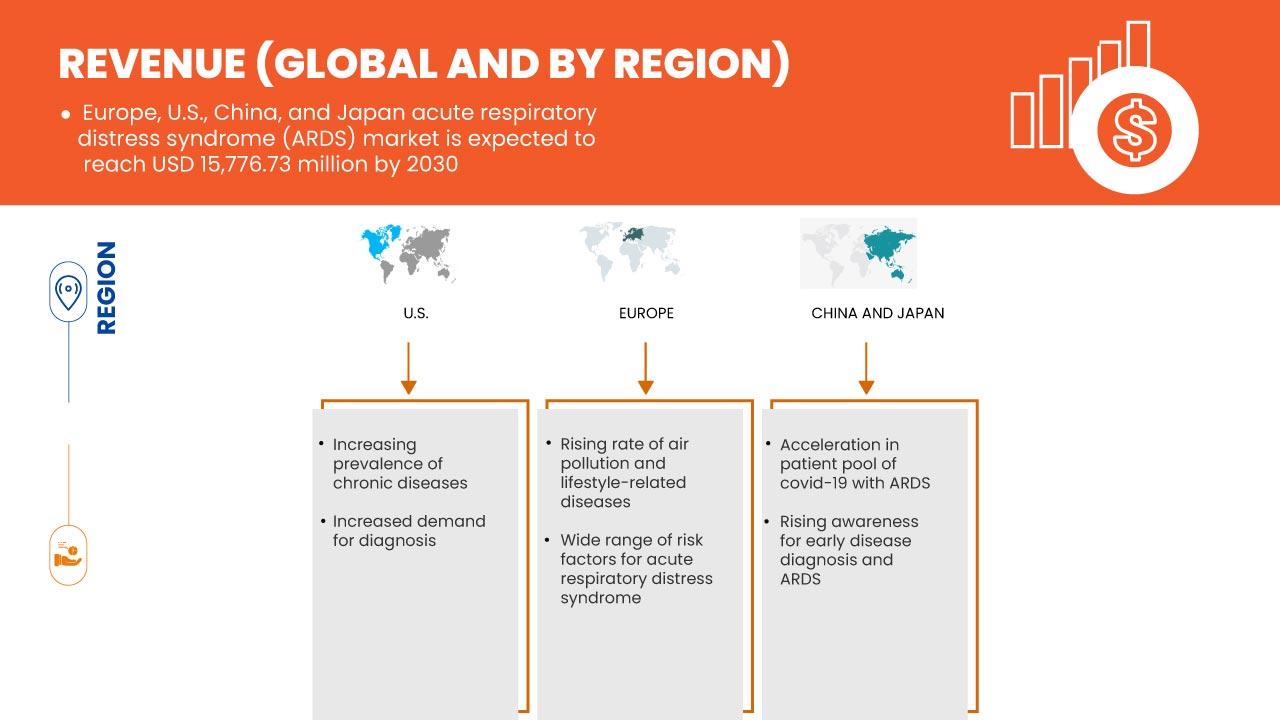
Le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est favorable et vise à réduire la maladie, améliorant ainsi la récupération et les performances des individus. Data Bridge Market Research analyse que le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon connaîtra un TCAC de 9,9 % au cours de la période de prévision de 2023 à 2030.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable pour 2020-2015) |
|
Unités quantitatives |
Chiffre d'affaires en millions, prix en USD |
|
Segments couverts |
Par cause (maladie à coronavirus 2019 (COVID-19), septicémie, inhalation de substances nocives, pneumonie grave et autres), type (diagnostic et traitement), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile et autres), canal de distribution (appel d'offres direct, pharmacie hospitalière, pharmacie de détail et pharmacie en ligne) |
|
Pays couvert |
États-Unis, Japon, Chine, Allemagne, Royaume-Uni, Italie, France, Espagne, Suisse, Russie, Turquie, Hongrie, Lituanie, Autriche, Irlande, Norvège, Pologne, Pays-Bas et reste de l'Europe |
|
Acteurs du marché couverts |
Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited, LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB. et EUROSETS, entre autres |
Définition du marché
Le syndrome de détresse respiratoire aiguë (SDRA) est une lésion pulmonaire potentiellement mortelle qui permet à du liquide de s'infiltrer dans les poumons. La plupart des personnes atteintes du SDRA sont hospitalisées pour un traumatisme ou une maladie comme la COVID-19. Le syndrome survient généralement lorsque des liquides s'accumulent dans les minuscules sacs aériens élastiques des poumons, appelés alvéoles. Cette accumulation de liquide entraîne une diminution de l'oxygène dans la circulation sanguine. Cela prive les organes de suffisamment d'oxygène pour leur fonctionnement normal. Les personnes atteintes d'une autre maladie développent un SDRA dans les quelques heures à quelques jours suivant la blessure ou l'infection qui l'a déclenchée. Le risque de décès augmente avec l'âge et, selon la gravité de la maladie, les patients qui survivent au syndrome deviennent difficiles. Une maladie ou une blessure grave endommageant les sacs membranaires des poumons conduit au SDRA. Les causes sous-jacentes les plus courantes de ces maladies comprennent la septicémie, l'inhalation de substances nocives, une pneumonie grave, une blessure à la tête, à la poitrine ou une autre blessure grave, la maladie à coronavirus 2019 (COVID-19) et d'autres.
Dynamique du marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon
Cette section traite de la compréhension des moteurs, des opportunités, des contraintes et des défis du marché. Tous ces éléments sont abordés en détail ci-dessous :
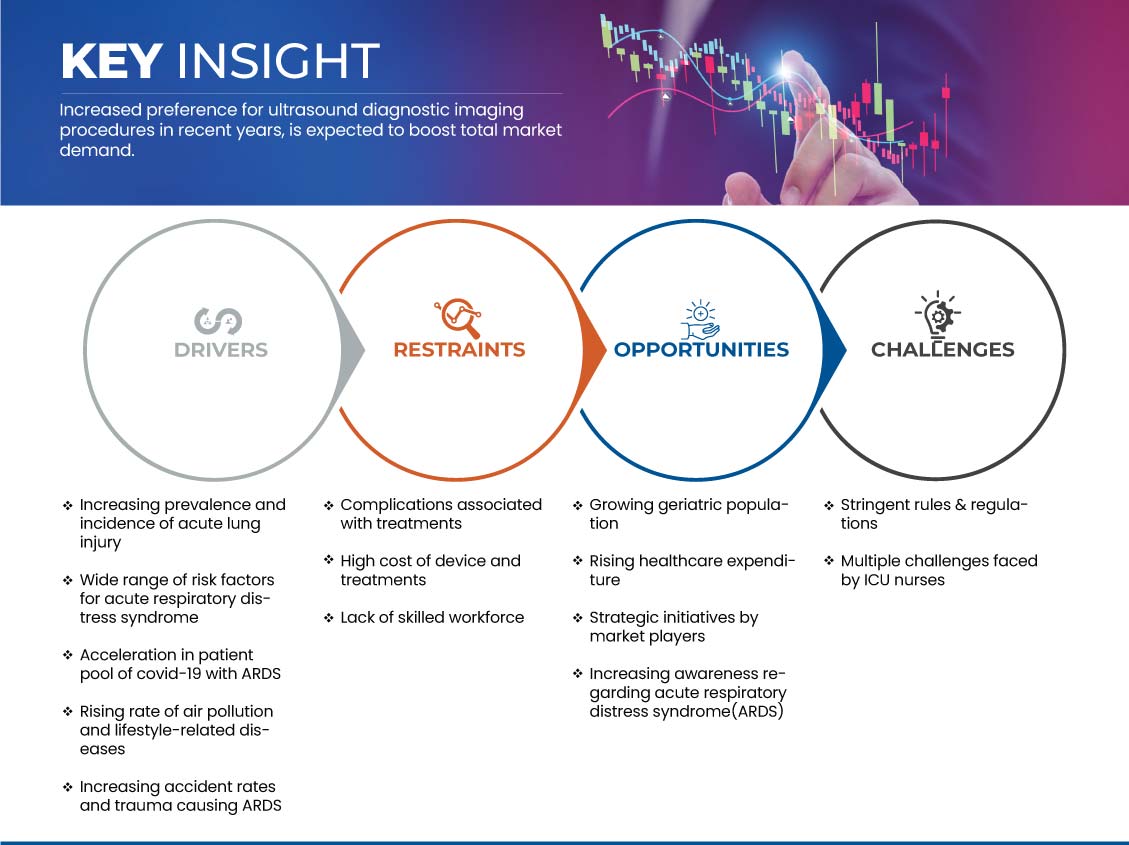
Conducteurs
- Augmentation de la prévalence et de l’incidence des lésions pulmonaires aiguës
Les cas de lésions pulmonaires aiguës sont de plus en plus signalés en raison de nombreux facteurs tels que le vieillissement de la population et l'augmentation du nombre de patients atteints de septicémie et de pneumonie. Cependant, la plupart des personnes ne reçoivent un diagnostic de lésions pulmonaires et de syndrome de détresse respiratoire aiguë qu'à un stade avancé. Cette maladie est une maladie à progression rapide qui survient chez les patients dont les poumons sont endommagés, provoquant une fuite de liquides corporels. Le nombre de cas de syndrome de détresse respiratoire aiguë et de lésions pulmonaires augmente en raison de l'émergence de divers virus responsables de maladies respiratoires ces dernières années, comme le COVID-19.
L'incidence et la prévalence du syndrome de détresse respiratoire aiguë ne cessent d'augmenter. Cette maladie est largement reconnue comme un problème clinique majeur dans le monde entier, entraînant une charge de morbidité et de mortalité élevée. Par conséquent, l'augmentation de la prévalence et des taux d'incidence des lésions pulmonaires aiguës et du syndrome de détresse respiratoire aiguë qui l'accompagne devrait stimuler le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon.
- Large éventail de facteurs de risque pour le syndrome de détresse respiratoire aiguë (SDRA)
Il existe une vaste gamme de facteurs de risque pour le syndrome de détresse respiratoire aiguë. Des facteurs de risque environnementaux et individuels sont impliqués dans le syndrome. Les patients atteints de SDRA souffrent de divers degrés de vasoconstriction des artères pulmonaires, ce qui entraîne des problèmes pour oxygéner le sang. Ils ont donc généralement besoin d'un respirateur pour respirer. Le SDRA entraîne une mortalité élevée et améliore cette maladie mortelle. Le syndrome septique avec défaillance multiviscérale est la cause la plus fréquente de décès, suivie de l'insuffisance respiratoire. De plus, la gravité du SDRA est associée de manière significative au taux de mortalité chez les patients gravement malades atteints de COVID-19.
Le SDRA peut être provoqué par de multiples causes, notamment un traumatisme. Les facteurs de risque de SDRA après un traumatisme multiple comprennent les lésions cérébrales et thoraciques traumatiques, la gravité et la durée du choc, le nombre de produits sanguins transfusés et les cristalloïdes perfusés.
Opportunité
- Sensibilisation accrue au syndrome de détresse respiratoire aiguë (SDRA)
Le syndrome de détresse respiratoire aiguë (SRA) a de multiples causes différentes et est généralement ignoré parmi les causes courantes de décès. La nécessité d'un traitement technique avancé et d'une bonne connaissance de la maladie peut réduire considérablement l'incidence du SRA. Comme un diagnostic et une prévention rapides sont essentiels pour prévenir ou guérir plus rapidement, l'attention du public est primordiale. Les gouvernements et les organisations actuels ont élargi le champ de recherche sur les lésions pulmonaires pour inclure la prévention primaire du SRA et réduire le taux de morbidité ou de mortalité du syndrome.
Les initiatives lancées il y a quelques années contribuent toujours à la prévention des infections pulmonaires graves comme le SDRA et aident les sociétés de biotechnologie et pharmaceutiques à innover dans leurs recherches pour de nouvelles avancées thérapeutiques. Bien qu'il n'existe pas de traitement spécifique ou approprié pour le SDRA, peu d'associations tentent de sensibiliser davantage les patients à ce syndrome et d'aider ces derniers à guérir rapidement de leurs poumons.
De tels programmes d'initiatives novateurs et des unités de soins de soutien lancés par diverses associations de soins de santé et de soins pulmonaires sensibilisent davantage les gens à la cause et à la gestion appropriée de la maladie à temps. Ainsi, une sensibilisation accrue au SDRA par le biais de diverses associations améliore les opportunités de croissance future du marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon.
Retenue/Défi
- Coût élevé des appareils et des traitements
Bien que le syndrome de détresse respiratoire aiguë bénéficie d'un large éventail d'options de traitement avancées, le coût du traitement à long terme est assez difficile à supporter pour les personnes à revenu moyen. Le recours aux services de soins intensifs et de réanimation augmente dans le monde entier, et son coût élevé constitue une préoccupation majeure dans le système de santé actuel. Les patients atteints du syndrome de détresse respiratoire aiguë doivent généralement subir de longues hospitalisations avec une monétarisation et un recours fréquents à la ventilation, ce qui consomme une quantité importante de ressources de santé. De ce fait, la plupart des patients qui ne peuvent pas se permettre un séjour de longue durée sont libérés dès les premières étapes du traitement. Cependant, cela augmente les possibilités et les susceptibilités à de nouvelles complications en cas d'infections, ce qui exige des ressources de santé et des traitements supplémentaires.
Impact post-COVID-19 sur le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon
La COVID-19 a eu un impact positif sur la croissance du marché, car la demande de produits contre le syndrome de détresse respiratoire aiguë a augmenté dans la région. Au cours de la phase de la COVID-19, il a été indiqué que plusieurs cas sont asymptomatiques, tandis que 20 % des cas de COVID-19 suivent une évolution grave, nécessitant une hospitalisation. Les cas graves de la maladie COVID-19 finiront par entraîner un SDRA et une pneumonie. Il a été prouvé que cela est mortel pour les personnes infectées. Comme le SDRA montre le défaut pulmonaire en endommageant les alvéoles, qui sont de minuscules sacs aériens dans les poumons, le même niveau de défaut a été observé chez les patients atteints de COVID-19. Cela entraîne un afflux soudain de liquide, provoquant une pneumonie. La COVID-19 a donc eu un impact positif sur ce marché.
Développements récents
- En mai 2021, Medtronic a lancé le système de surveillance des voies respiratoires SonarMed. Le système utilise la technologie acoustique pour vérifier l'obstruction du tube endotrachéal. Cela a aidé l'entreprise à élargir son portefeuille de produits
- En juillet 2020, F. Hoffman-La Roche Ltd a lancé un test rapide d'anticorps contre le SARS-CoV-2. Le test a été lancé en partenariat avec SD Biosenseor, Inc. Cela a aidé l'entreprise à élargir son portefeuille de produits
Portée du marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon
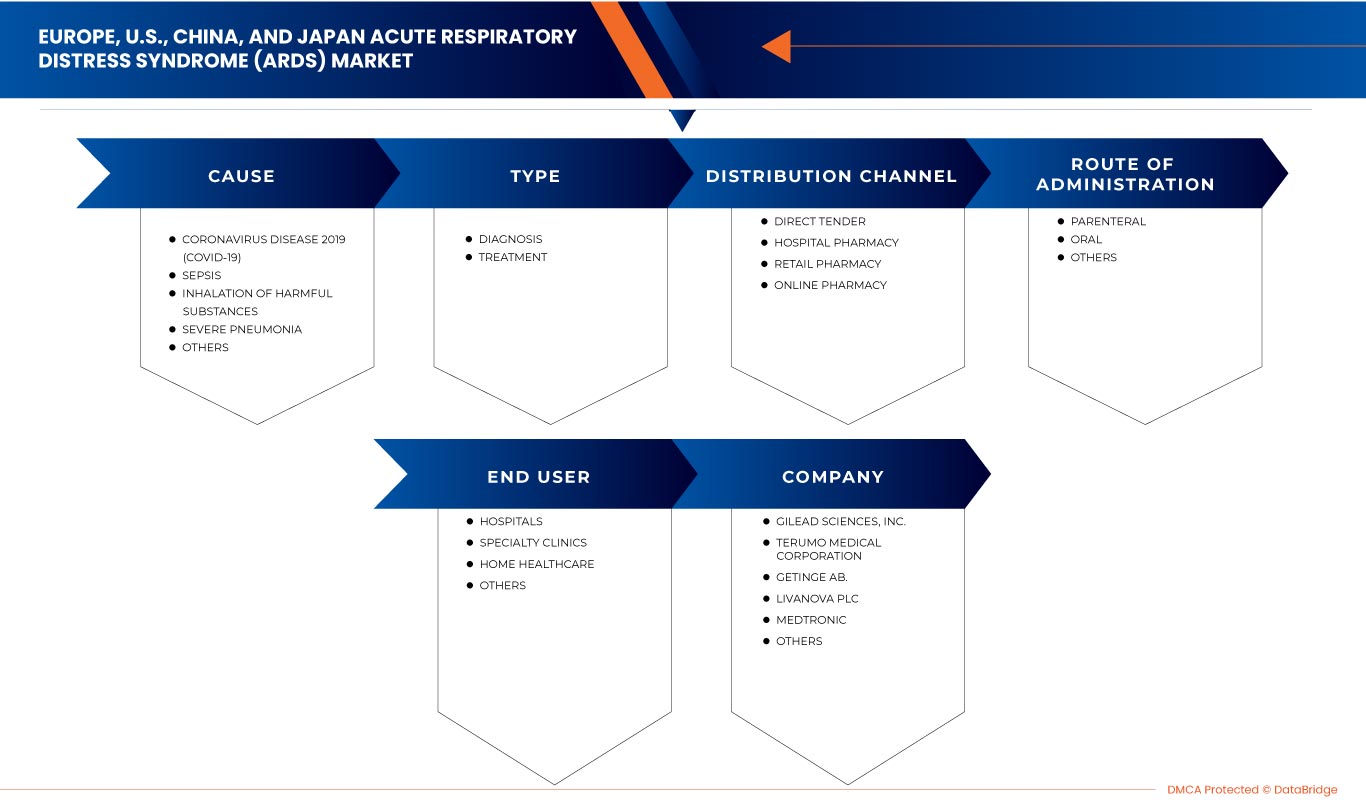
Le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est classé en cinq segments en fonction de la cause, du type, de la voie d'administration, de l'utilisateur final et du canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
Cause
- Maladie à coronavirus 2019 (COVID-19)
- État septique
- Inhalation de substances nocives
- Pneumonie grave
- Autres
En fonction de la cause, le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est segmenté en maladie à coronavirus 2019 (COVID-19), septicémie, inhalation de substances nocives, pneumonie sévère et autres.
Taper
- Diagnostic
- Traitement
En fonction du type, le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est segmenté en diagnostic et traitement.
Voie d'administration
- Oral
- Parentérale
- Autres
En fonction de la voie d'administration, le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est segmenté en voie orale, parentérale et autres.
Utilisateur final
- Hôpitaux
- Cliniques spécialisées
- Soins à domicile
- Autres
En fonction de l'utilisateur final, le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est segmenté en hôpitaux, cliniques spécialisées, soins de santé à domicile et autres.
Canal de distribution
- Appel d'offres direct
- Pharmacie de l'hôpital
- Pharmacie de détail
- Pharmacie en ligne
En fonction du canal de distribution, le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est segmenté en appel d'offres direct, pharmacie hospitalière, pharmacie de détail et pharmacie en ligne.
Analyse/perspectives du marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon
Le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon est analysé, et des informations et tendances sur la taille du marché sont fournies par la cause, le type, la voie d’administration, l’utilisateur final et le canal de distribution comme référencé ci-dessus.
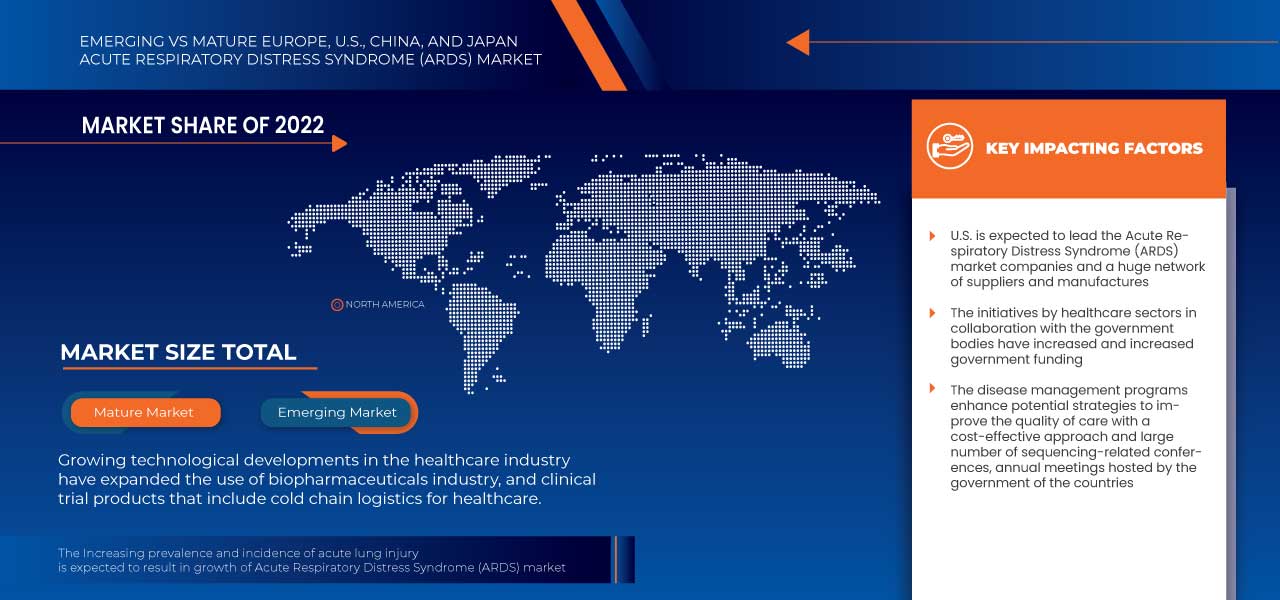
Les pays couverts par le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon sont les États-Unis, le Japon, la Chine, l'Allemagne, le Royaume-Uni, l'Italie, la France, l'Espagne, la Suisse, la Russie, la Turquie, la Hongrie, la Lituanie, l'Autriche, l'Irlande, la Norvège, la Pologne, les Pays-Bas et le reste de l'Europe.
Le marché américain du syndrome de détresse respiratoire aiguë (SDRA) devrait croître en raison d’une augmentation de la prévalence des lésions pulmonaires aiguës ainsi que d’une augmentation du nombre de patients atteints de COVID-19 avec SDRA. Ce sont les principaux facteurs qui devraient stimuler la croissance du marché dans le pays.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que l'analyse de la chaîne de valeur en aval et en amont, les tendances techniques, l'analyse des cinq forces du porteur et les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario de marché pour chaque pays. En outre, la présence et la disponibilité des marques et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales et l'impact des tarifs nationaux et des routes commerciales sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon
Le paysage concurrentiel du marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon fournit des détails sur les concurrents. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, la présence, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation de l'entreprise sur le marché du syndrome de détresse respiratoire aiguë (SDRA) en Europe, aux États-Unis, en Chine et au Japon.
Français Certains des principaux acteurs opérant sur le marché sont Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB., et EUROSETS, entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 ETIOLOGY BY GEOGRAPHY
4.3.1 ETIOLOGY IN U.S.
4.3.2 ETIOLOGY IN EUROPE
4.3.3 ETIOLOGY IN CHINA
4.3.4 ETIOLOGY IN JAPAN
4.4 ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) HEALTHCARE COST PER PATIENT BY GEOGRAPHY
4.5 INSURANCE REIMBURSEMENT
4.5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
4.5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
4.5.3 ABBOTT CODING GUIDE FOR ECMO
4.5.4 CENTRAL GOVERNMENT HEALTH SCHEME (CGHS)
4.5.5 CERN HEALTH INSURANCE SCHEME
4.5.6 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
4.5.7 AMERICAN HOSPITAL ASSOCIATION
4.5.8 CONCLUSION
4.6 PIPELINE ANALYSIS
5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
5.1 REGULATION IN U.S.:
5.2 LABELING OF MODIFIED DEVICES
5.3 REGULATION IN EUROPE:
5.4 REGULATION IN CHINA:
5.5 REGULATION IN JAPAN:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
6.1.2 WIDE RANGE OF RISK FACTORS FOR ACUTE RESPIRATORY DISTRESS SYNDROME
6.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
6.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
6.1.5 INCREASING ACCIDENT RATES AND TRAUMA-CAUSING ARDS
6.2 RESTRAINTS
6.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
6.2.2 HIGH COST OF DEVICE AND TREATMENTS
6.2.3 LACK OF SKILLED WORKFORCE
6.3 OPPORTUNITIES
6.3.1 GROWING GERIATRIC POPULATION
6.3.2 RISING HEALTHCARE EXPENDITURE
6.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.4 INCREASING AWARENESS REGARDING ACUTE RESPIRATORY DISTRESS SYNDROME(ARDS)
6.4 CHALLENGES
6.4.1 STRINGENT RULES & REGULATIONS
6.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
7.1 OVERVIEW
7.2 CORONAVIRUS DISEASE 2019 (COVID-19)
7.3 SEPSIS
7.4 INHALATION OF HARMFUL SUBSTANCES
7.5 SEVERE PNEUMONIA
7.6 OTHERS
8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
8.1 OVERVIEW
8.2 DIAGNOSIS
8.2.1 IMAGING TESTS
8.2.1.1 CHEST X-RAY
8.2.1.2 CT SCAN
8.2.1.3 ULTRASOUND
8.2.1.4 OTHERS
8.2.2 BLOOD TEST
8.2.3 RESPIRATORY RATE
8.2.4 SPO2 TEST
8.2.5 OTHERS
8.3 TREATMENT
8.3.1 MECHANICAL VENTILATION
8.3.1.1 HIGH-FLOW NASAL O2
8.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
8.3.1.3 CONTINUOUS POSITIVE AIRWAY PRESSURE
8.3.1.4 PRONE POSITIVE VENTILATION
8.3.1.5 OTHERS
8.3.2 CORTICOSTEROIDS
8.3.2.1 METHYLPREDNISOLONE
8.3.2.2 DEXAMETHASONE
8.3.2.3 OTHERS
8.3.3 ANTIVIRAL MEDICATION
8.3.3.1 REMDESIVIR
8.3.3.2 COMBINATION DRUGS
8.3.3.3 OTHERS
8.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
8.3.5 TOCILIZUMAB
8.3.6 OTHERS
9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.2.1 INTRAVENOUS
9.2.2 INTRAMUSCULAR
9.3 ORAL
9.4 OTHERS
10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOME HEALTHCARE
10.5 OTHERS
11 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 HOSPITAL PHARMACY
11.4 RETAIL PHARMACY
11.5 ONLINE PHARMACY
12 EUROPE
12.1 GERMANY
12.2 FRANCE
12.3 U.K.
12.4 ITALY
12.5 SPAIN
12.6 TURKEY
12.7 HUNGARY
12.8 NETHERLANDS
12.9 SWITZERLAND
12.1 AUSTRIA
12.11 LITHUANIA
12.12 POLAND
12.13 RUSSIA
12.14 IRELAND
12.15 NORWAY
12.16 REST OF EUROPE
13 EUROPE, US, CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: U.S.
13.2 COMPANY SHARE ANALYSIS: EUROPE
13.3 COMPANY SHARE ANALYSIS: JAPAN
13.4 COMPANY SHARE ANALYSIS: CHINA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 GILEAD SCIENCES INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUS ANALYSIS
15.1.3 PRODUCT PORTFOLIO
15.1.4 RECENT DEVELOPMENT
15.2 TERUMO CORPORATION
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUS ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 GETINGE AB
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUS ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 LIVANOVA PLC
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 PRODUCT PORTFOLIO
15.4.4 RECENT DEVELOPMENTS
15.5 MEDTRONIC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENTS
15.6 ALUNG TECHNOLOGIES, INC
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 ARMSTRONG MEDICAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 BESMED HEALTH BUSINESS CORP.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 DRÄGERWERK AG & CO. KGAA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 EUROSETS
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 F. HOFFMANN-LA ROCHE LTD
15.11.1 COMPANY SNAPSHOT
15.11.2 RECENT ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.12 FISHER & PAYKEL HEALTHCARE LIMITED
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENTS
15.13 FRESENIUS SE & CO. KGAA
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUS ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HAMILTON MEDICAL
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 NICE NEOTECH MEDICAL SYSTEMS PVT.LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NIPRO
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUS ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 PFIZER INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUS ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 RESMED
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 SMITHS MEDICAL
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUS ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Liste des tableaux
TABLE 1 HEALTHCARE COST FOR ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) ON THE BASIS OF SEVERITY BY COUNTRY IS GIVEN BELOW IN USD:
TABLE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 3 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
TABLE 4 REGULATION FOR THE USE OF VENTILATOR AND ANESTHESIA GAS MACHINE BREATHING CIRCUIT DEVICES
TABLE 5 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 7 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 8 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 14 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 CHINA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 16 JAPAN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 17 EUROPE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 18 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 CHINA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 20 JAPAN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 21 EUROPE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030(USD MILLION)
TABLE 22 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 23 CHINA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 24 JAPAN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 25 EUROPE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 27 CHINA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 EUROPE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 CHINA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 33 EUROPE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 34 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 CHINA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 36 JAPAN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 40 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 EUROPE PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 U.S. PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 43 CHINA PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 44 JAPAN PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 45 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 49 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 50 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 52 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 54 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 55 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 56 GERMANY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 57 GERMANY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 58 GERMANY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 59 GERMANY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 60 GERMANY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 61 GERMANY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 62 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 63 GERMANY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 64 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 66 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 67 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 FRANCE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 69 FRANCE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 FRANCE PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 76 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 79 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.K. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.K. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.K. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.K. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.K. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.K. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 86 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 87 U.K. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 88 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 89 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 90 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 91 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 92 ITALY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 93 ITALY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 94 ITALY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 95 ITALY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 96 ITALY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 97 ITALY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 98 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 99 ITALY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 100 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 101 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 102 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 103 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 104 SPAIN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 105 SPAIN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 106 SPAIN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 107 SPAIN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 108 SPAIN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 109 SPAIN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SPAIN PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 115 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 TURKEY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 TURKEY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 TURKEY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 TURKEY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 120 TURKEY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 121 TURKEY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 122 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 123 TURKEY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION
TABLE 124 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 125 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 126 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 127 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 128 HUNGARY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 129 HUNGARY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 130 HUNGARY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 HUNGARY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 132 HUNGARY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 HUNGARY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 135 HUNGARY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 136 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 137 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 138 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 139 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 140 NETHERLANDS DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 141 NETHERLANDS IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 142 NETHERLANDS TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 143 NETHERLANDS ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 NETHERLANDS CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 145 NETHERLANDS MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 146 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 NETHERLANDS PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 148 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 149 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 150 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 151 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 SWITZERLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 SWITZERLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 SWITZERLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 SWITZERLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 156 SWITZERLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 157 SWITZERLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 158 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 SWITZERLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 163 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 164 AUSTRIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 165 AUSTRIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 AUSTRIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 167 AUSTRIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 AUSTRIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 AUSTRIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 171 AUSTRIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 172 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 173 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 174 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 175 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 LITHUANIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 177 LITHUANIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 178 LITHUANIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 LITHUANIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 180 LITHUANIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 181 LITHUANIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 183 LITHUANIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 184 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 185 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 186 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 187 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 POLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 POLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 POLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 191 POLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 192 POLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 193 POLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 194 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 POLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 197 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 198 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 199 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 200 RUSSIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 201 RUSSIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 202 RUSSIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 203 RUSSIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 RUSSIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 RUSSIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 207 RUSSIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 208 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 209 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 210 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 211 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 212 IRELAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 213 IRELAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 214 IRELAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 215 IRELAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 216 IRELAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 217 IRELAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 218 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 219 IRELAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 220 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 221 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 222 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 223 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 224 NORWAY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 225 NORWAY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 226 NORWAY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 227 NORWAY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 228 NORWAY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 229 NORWAY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 230 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 231 NORWAY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 232 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 233 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 234 REST OF EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN THE PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 13 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S.ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 14 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 15 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 16 MOST COMMON PRIMARY CAUSES OF DEATH IN ARDS PATIENTS IN U.S. COUNTRY
FIGURE 17 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 18 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 19 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 21 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 26 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 27 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 28 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 29 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 30 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 31 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 32 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 33 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 34 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 35 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 36 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 39 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 40 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 41 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 42 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 43 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 44 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 45 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 46 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 47 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 49 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 50 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 51 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 52 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 54 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 55 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 56 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 57 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 58 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 59 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 60 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 61 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 62 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 63 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 64 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 65 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 66 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 67 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 68 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 69 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 70 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 71 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 72 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 73 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 74 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 75 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 76 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 77 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 78 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 79 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 80 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 81 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 82 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 83 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 84 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 85 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 86 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 87 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 88 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 89 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 90 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 91 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 92 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 93 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 94 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 95 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 96 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 97 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 98 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SNAPSHOT (2022)
FIGURE 99 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022)
FIGURE 100 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2023 & 2030)
FIGURE 101 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022 & 2030)
FIGURE 102 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: CAUSE (2023-2030)
FIGURE 103 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 104 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 105 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 106 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.