Europe Molecular Diagnostics Services Market
Taille du marché en milliards USD
TCAC :
% 
 USD
38.02 Million
USD
67.31 Million
2024
2032
USD
38.02 Million
USD
67.31 Million
2024
2032
| 2025 –2032 | |
| USD 38.02 Million | |
| USD 67.31 Million | |
|
|
|
|
Segmentation du marché européen des services de diagnostic moléculaire, par type de service (réparation d'instruments, formation, conformité, étalonnage, maintenance, automatisation évolutive, clés en main, relocalisation d'instruments, personnalisation du matériel, assurance de la performance, conception et développement, solutions de chaîne d'approvisionnement, lancement de nouveaux produits, fabrication, environnement et réglementation, certification et audit des systèmes de gestion médicale, recherche clinique, conseil et autres), technologie (PCR, PCR en temps réel, séquençage nouvelle génération et autres technologies), utilisateur final (hôpitaux, centres de diagnostic, établissements universitaires et de recherche, etc.) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché européen des services de diagnostic moléculaire
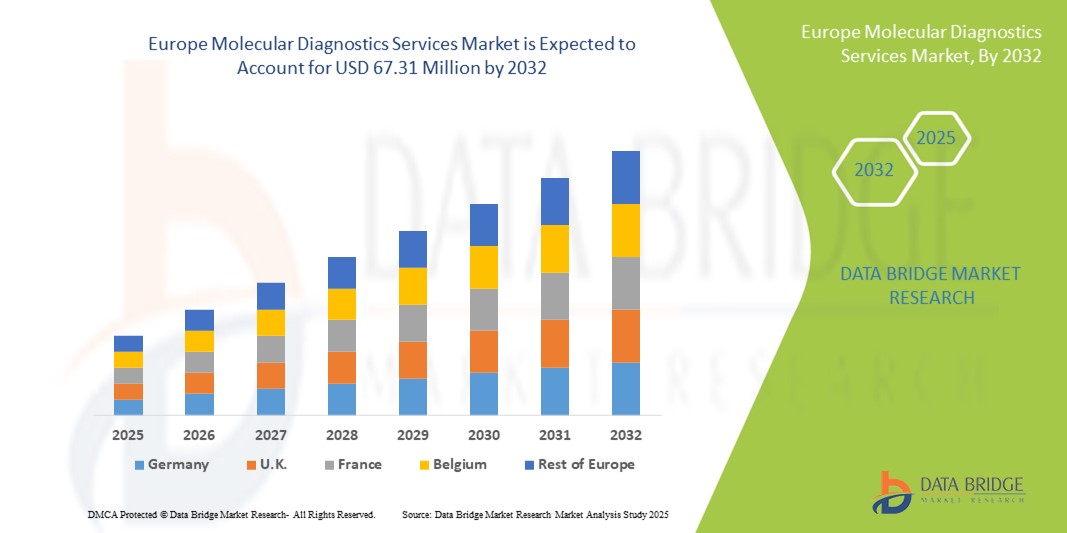
- La taille du marché européen des services de diagnostic moléculaire était évaluée à 38,02 millions USD en 2024 et devrait atteindre 67,31 millions USD d'ici 2032 , à un TCAC de 7,40 % au cours de la période de prévision.
- La croissance du marché est largement tirée par la prévalence croissante des maladies chroniques et infectieuses , l'adoption croissante de la médecine personnalisée et les avancées technologiques dans les techniques de diagnostic moléculaire, telles que la PCR , le NGS et les tests basés sur des puces à ADN.
- Par ailleurs, la demande croissante de solutions de détection précoce des maladies, de diagnostic précis et de tests à haut débit dans les hôpitaux, les laboratoires de diagnostic et les centres de recherche positionne les services de diagnostic moléculaire comme un élément essentiel des soins de santé modernes. Ces facteurs convergents accélèrent leur adoption par le marché, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché européen des services de diagnostic moléculaire
- Les services de diagnostic moléculaire, englobant des techniques avancées telles que la PCR, la PCR en temps réel et le séquençage de nouvelle génération , deviennent de plus en plus essentiels dans l'écosystème des soins de santé en Europe en raison de leur rôle dans la détection précise des maladies, les applications de recherche et l'optimisation des flux de travail en laboratoire dans les hôpitaux, les centres de diagnostic et les établissements universitaires.
- La demande croissante de services de diagnostic moléculaire est principalement motivée par la prévalence croissante des maladies chroniques et infectieuses, l'adoption croissante de la médecine de précision et les progrès technologiques permettant des tests plus rapides, plus fiables et plus rentables.
- L'Allemagne a dominé le marché des services de diagnostic moléculaire avec la plus grande part de revenus de 39,6 % en 2024, soutenue par une infrastructure de soins de santé avancée, une forte adoption des technologies moléculaires et une forte présence de fournisseurs de services de premier plan, avec une adoption significative dans les hôpitaux, les centres de diagnostic et les instituts de recherche.
- La Pologne devrait être le pays connaissant la croissance la plus rapide sur le marché des services de diagnostic moléculaire au cours de la période de prévision en raison de l'augmentation des investissements dans les soins de santé, de l'expansion des capacités des laboratoires et de la demande croissante de services complets tels que la maintenance, l'étalonnage et les solutions clés en main.
- Le segment des services de maintenance a dominé le marché des services de diagnostic moléculaire en 2024 avec une part de marché de 29,7 %, grâce au besoin croissant d'opérations de laboratoire ininterrompues, d'une grande fiabilité des instruments de diagnostic moléculaire et de solutions de service rentables dans les milieux cliniques et de recherche.
Portée du rapport et segmentation du marché européen des services de diagnostic moléculaire
|
Attributs |
Informations clés sur le marché des services de diagnostic moléculaire en Europe |
|
Segments couverts |
|
|
Pays couverts |
Europe
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché européen des services de diagnostic moléculaire
Automatisation avancée et solutions de laboratoire intégrées
- Une tendance significative et croissante sur le marché européen des services de diagnostic moléculaire est l'adoption croissante de solutions de laboratoire automatisées et intégrées qui améliorent l'efficacité du flux de travail et réduisent les erreurs manuelles.
- Par exemple, les plates-formes d'automatisation évolutives permettent la gestion simultanée de plusieurs échantillons, améliorant ainsi le débit tout en maintenant la précision des tests PCR, PCR en temps réel et NGS.
- L'intégration de systèmes de gestion de laboratoire pilotés par logiciel avec des instruments de diagnostic permet une surveillance en temps réel des performances des instruments et le suivi des échantillons, réduisant ainsi les goulots d'étranglement opérationnels
- Une telle automatisation permet également une maintenance prédictive et une détection des erreurs, garantissant une prestation de services ininterrompue et minimisant les temps d'arrêt du laboratoire.
- Les prestataires de services tels qu'Eurofins et SGS développent des solutions de bout en bout qui combinent l'automatisation avec des services de conseil et de formation, améliorant ainsi l'efficacité et la conformité des laboratoires.
- La tendance vers des laboratoires de diagnostic moléculaire intelligents, entièrement intégrés et automatisés remodèle les normes opérationnelles, stimulant la demande de packages de services complets dans les hôpitaux et les centres de diagnostic.
Dynamique du marché européen des services de diagnostic moléculaire
Conducteur
Demande croissante en raison de la charge croissante de morbidité et de la médecine personnalisée
- La prévalence croissante des maladies chroniques et infectieuses, associée à l’adoption croissante de la médecine personnalisée, constitue un facteur important de la demande accrue de services de diagnostic moléculaire.
- Par exemple, les hôpitaux et les centres de diagnostic s'appuient de plus en plus sur les tests PCR et NGS pour la détection précoce des troubles génétiques et des agents pathogènes infectieux.
- La sensibilisation croissante des professionnels de la santé et des patients aux diagnostics de précision alimente la demande de solutions de test plus rapides, plus précises et plus fiables.
- L'expansion des infrastructures de soins de santé, en particulier dans des pays comme l'Allemagne, permet une plus grande disponibilité des services de diagnostic moléculaire dans les milieux cliniques et de recherche.
- Des offres de services complètes, comprenant la maintenance, l'étalonnage et la formation, améliorent la productivité du laboratoire et l'adoption de technologies de diagnostic avancées
- Le besoin de solutions de diagnostic moléculaire rapides, précises et évolutives oblige les prestataires de soins de santé à intégrer ces services comme pratique standard dans les protocoles de gestion des maladies.
Retenue/Défi
Coûts élevés et défis en matière de conformité réglementaire
- Les préoccupations concernant les coûts d’exploitation et de service élevés constituent un défi important pour une pénétration plus large du marché, en particulier pour les laboratoires de petite et moyenne taille.
- Par exemple, les plates-formes d’automatisation avancées et les services basés sur NGS nécessitent un investissement initial important, ce qui limite leur adoption dans les installations à budget limité.
- Les exigences strictes en matière de réglementation et de conformité qualité en Europe nécessitent une documentation, une validation et une certification solides, ce qui augmente la complexité opérationnelle
- Relever les défis de conformité tout en garantissant une prestation de services de haute qualité est essentiel pour gagner la confiance des laboratoires et des institutions
- La variabilité des prix des services et la disponibilité limitée de personnel qualifié pour les procédures de diagnostic moléculaire complexes peuvent limiter la croissance du marché dans certaines régions.
- Surmonter ces défis grâce à l’optimisation des coûts, au soutien réglementaire et aux programmes de formation du personnel de laboratoire sera essentiel pour une adoption durable et l’expansion du marché.
Portée du marché européen des services de diagnostic moléculaire
Le marché est segmenté en fonction du type de service, de la technologie et de l’utilisateur final.
- Par type de service
En fonction du type de service, le marché européen des services de diagnostic moléculaire est segmenté en services de réparation d'instruments, services de formation, services de conformité, services d'étalonnage, services de maintenance, services d'automatisation évolutive, services clés en main, services de relocalisation d'instruments, personnalisation du matériel, services d'assurance de la performance, services de conception et développement, solutions de chaîne d'approvisionnement, services de lancement de nouveaux produits, services de fabrication, services environnementaux et réglementaires, certification et audit des systèmes de gestion médicale, services de recherche clinique, services de conseil, etc. Le segment des services de maintenance a dominé le marché avec la plus grande part de chiffre d'affaires (29,7 %) en 2024, stimulé par le besoin croissant d'assurer la continuité des opérations des laboratoires. Les hôpitaux, les centres de diagnostic et les instituts de recherche privilégient les services de maintenance pour éviter les temps d'arrêt coûteux et garantir une fiabilité élevée des instruments de diagnostic moléculaire. La demande est également soutenue par la complexité des flux de travail des plateformes PCR, PCR en temps réel et NGS, où la surveillance continue des performances est essentielle. Les prestataires de services associent également la maintenance aux services d'étalonnage et de formation, améliorant ainsi la proposition de valeur globale. L'augmentation des investissements dans les infrastructures de laboratoire en Allemagne et sur d'autres marchés matures renforce la domination des services de maintenance. Les solutions de maintenance de routine et préventive sont de plus en plus privilégiées pour des raisons de rentabilité et de conformité réglementaire.
Le segment des services clés en main devrait connaître la croissance la plus rapide, soit 24 % entre 2025 et 2032, grâce à une adoption croissante sur les marchés émergents comme la Pologne. Les services clés en main offrent des solutions complètes incluant l'installation, la conception des flux de travail et la formation, allégeant ainsi la charge de travail des laboratoires dans la gestion de processus de mise en œuvre complexes. Ces services sont particulièrement attractifs pour les hôpitaux et les centres de diagnostic qui adoptent pour la première fois de nouvelles technologies moléculaires. La flexibilité, l'efficacité et la réduction du risque opérationnel offertes par les services clés en main favorisent une adoption rapide. La préférence croissante pour des solutions complètes et prêtes à l'emploi accélère la croissance des laboratoires cliniques et de recherche.
- Par technologie
Sur le plan technologique, le marché européen des services de diagnostic moléculaire est segmenté en PCR, PCR en temps réel, séquençage de nouvelle génération (NGS) et autres technologies. Le segment PCR a dominé le marché avec une part de chiffre d'affaires de 35 % en 2024, grâce à son utilisation répandue pour la détection de routine des maladies infectieuses, les tests génétiques et les applications de recherche. La PCR est extrêmement fiable, rentable et s'appuie sur des protocoles de service établis pour l'étalonnage, la maintenance et la réparation. De nombreux centres de diagnostic et hôpitaux s'appuient sur les tests basés sur la PCR pour leurs délais d'exécution rapides et leur précision, ce qui en fait l'épine dorsale du diagnostic moléculaire en Europe. L'Allemagne et d'autres pays d'Europe occidentale affichent une forte adoption grâce à des infrastructures de santé avancées et à des réseaux de laboratoires bien établis. L'intégration avec les services de maintenance, d'étalonnage et d'automatisation renforce encore la position dominante de la PCR. La grande connaissance clinique des flux de travail PCR garantit une demande continue auprès des utilisateurs finaux.
Le segment du séquençage de nouvelle génération (NGS) devrait connaître le TCAC le plus rapide, de 23 à 25 % entre 2025 et 2032, grâce à une adoption croissante pour la médecine personnalisée, les tests oncologiques et les applications de recherche. Le NGS permet un séquençage à haut débit, fournissant des informations génétiques complètes pour le diagnostic des maladies et le choix des thérapies. Cette croissance est soutenue par des investissements croissants dans la formation, le conseil et les services clés en main pour aider les laboratoires à mettre en œuvre les flux de travail du NGS. La Pologne et d'autres marchés européens émergents adoptent de plus en plus le NGS en raison d'une prise de conscience croissante des avantages de la médecine de précision. Les améliorations technologiques continues et la baisse des coûts du séquençage accélèrent son adoption. Le NGS devient une technologie privilégiée pour les diagnostics complexes nécessitant des informations génomiques complètes.
- Par utilisateur final
En fonction de l'utilisateur final, le marché européen des services de diagnostic moléculaire est segmenté entre hôpitaux, centres de diagnostic, établissements universitaires et de recherche, et autres. Le segment hospitalier a dominé le marché avec une part de chiffre d'affaires de 40 % en 2024, portée par ses besoins importants en matière de diagnostic et sa demande de tests moléculaires fiables. Les hôpitaux ont besoin d'offres de services complètes, incluant la maintenance, l'étalonnage, la formation et des solutions clés en main, pour garantir des opérations de laboratoire précises et ininterrompues. L'Allemagne et d'autres marchés européens matures disposent de réseaux hospitaliers bien établis qui adoptent des technologies de diagnostic moléculaire avancées telles que la PCR, la PCR en temps réel et le NGS. L'intégration des services aux systèmes de gestion des laboratoires hospitaliers améliore l'efficacité des flux de travail et réduit les temps d'arrêt opérationnels. L'exigence de conformité des hôpitaux aux normes réglementaires renforce encore la demande de solutions complètes. L'augmentation des investissements dans des laboratoires de diagnostic moléculaire modernes maintient les hôpitaux comme segment d'utilisateurs finaux dominant.
Le segment des institutions académiques et de recherche devrait connaître la croissance la plus rapide, soit 23 % entre 2025 et 2032, grâce au développement des activités de recherche et au financement de la génomique, des maladies infectieuses et de la recherche translationnelle. Les institutions de recherche adoptent de plus en plus l'automatisation évolutive, le NGS et les services clés en main pour accélérer les délais d'étude et améliorer la fiabilité des données. Les marchés émergents comme la Pologne investissent dans des infrastructures de recherche de pointe, créant une forte demande de services de diagnostic moléculaire. Les institutions académiques bénéficient de prestataires de services proposant des services de formation, de conseil et de conception et développement pour des applications spécialisées. Cette croissance est également soutenue par des initiatives de recherche collaborative entre universités et hôpitaux, favorisant l'adoption de technologies moléculaires de pointe.
Analyse régionale du marché européen des services de diagnostic moléculaire
- L'Allemagne a dominé le marché des services de diagnostic moléculaire avec la plus grande part de revenus de 39,6 % en 2024, soutenue par une infrastructure de soins de santé avancée, une forte adoption des technologies moléculaires et une forte présence de fournisseurs de services de premier plan, avec une adoption significative dans les hôpitaux, les centres de diagnostic et les instituts de recherche.
- Les hôpitaux, les centres de diagnostic et les instituts de recherche en Allemagne privilégient la précision, la fiabilité et des offres de services complètes, notamment la maintenance, l'étalonnage, la formation et les solutions clés en main pour les plateformes PCR, PCR en temps réel et NGS.
- Cette adoption généralisée est en outre soutenue par des dépenses de santé élevées, des cadres de conformité réglementaire solides et des investissements croissants dans la modernisation des laboratoires, établissant les services de diagnostic moléculaire comme un élément essentiel des opérations cliniques et de recherche à travers le pays.
Aperçu du marché allemand des services de diagnostic moléculaire
Le marché allemand des services de diagnostic moléculaire domine le marché européen avec une part de chiffre d'affaires de 39,6 % en 2024, portée par une infrastructure de santé de pointe, une adoption massive des technologies moléculaires telles que la PCR, la PCR en temps réel et le NGS, et une demande croissante de diagnostics de précision. Les hôpitaux, les centres de diagnostic et les instituts de recherche privilégient des tests fiables, précis et à haut débit, soutenus par des services complets comprenant la maintenance, l'étalonnage, la formation et des solutions clés en main. L'accent mis par le pays sur l'innovation, la conformité réglementaire et la modernisation des laboratoires renforce l'adoption, tandis que la présence de prestataires de services de premier plan garantit un accès généralisé aux technologies de diagnostic moléculaire de pointe. La domination de l'Allemagne reflète sa maturité technologique, ses réseaux de laboratoires bien établis et ses investissements importants dans les infrastructures de santé et de recherche, ce qui en fait un pôle clé pour les services de diagnostic moléculaire en Europe.
Aperçu du marché polonais des services de diagnostic moléculaire
Le marché polonais des services de diagnostic moléculaire devrait connaître la croissance la plus rapide d'Europe au cours de la période de prévision, grâce à la hausse des investissements dans le secteur de la santé, au développement des capacités des laboratoires et à l'adoption croissante de technologies avancées de diagnostic moléculaire. Les hôpitaux, les centres de diagnostic et les instituts de recherche s'appuient de plus en plus sur des services tels que la maintenance, l'étalonnage, les solutions clés en main et la formation pour mettre en œuvre efficacement les plateformes PCR, PCR en temps réel et NGS. Cette croissance est soutenue par la modernisation des infrastructures de laboratoire, la sensibilisation accrue à la médecine de précision et l'amélioration de la conformité aux normes réglementaires européennes. Les nouveaux centres de santé et de recherche polonais accélèrent l'adoption des services de diagnostic moléculaire, faisant de ce marché un marché en pleine expansion en Europe.
Analyse du marché français des services de diagnostic moléculaire
Le marché français des services de diagnostic moléculaire connaît une croissance soutenue, soutenue par des infrastructures de santé de haute qualité, l'adoption croissante des technologies moléculaires et l'accent mis sur la détection précoce des maladies. Les hôpitaux et centres de diagnostic français intègrent de plus en plus de services tels que la maintenance, l'étalonnage, la formation et le conseil afin d'améliorer l'efficacité des laboratoires et de garantir des résultats d'analyse précis. L'importance accordée par le pays à la recherche, à la conformité réglementaire et à l'accréditation des laboratoires stimule la demande de prestataires de services professionnels. La France adopte également des solutions innovantes, telles que des services d'automatisation clés en main et évolutifs, pour rationaliser les flux de travail et réduire les difficultés opérationnelles. La sensibilisation croissante à la médecine de précision et à la gestion des maladies chroniques favorise également leur adoption. Le marché bénéficie d'initiatives gouvernementales fortes en faveur de la modernisation des soins de santé et de la normalisation des laboratoires.
Aperçu du marché britannique des services de diagnostic moléculaire
Le marché britannique des services de diagnostic moléculaire devrait connaître une croissance annuelle moyenne (TCAC) notable au cours de la période de prévision, stimulé par la prévalence croissante des maladies infectieuses et génétiques, l'adoption croissante de technologies moléculaires avancées et la demande de solutions diagnostiques précises. Les hôpitaux, les centres de diagnostic et les instituts de recherche investissent dans des services complets, incluant la maintenance, l'étalonnage, la formation et des solutions clés en main, afin d'améliorer la productivité des laboratoires. La solidité du système de santé britannique, les initiatives de recherche et les cadres réglementaires favorisent l'adoption par le marché. L'intégration du diagnostic moléculaire aux programmes de médecine de précision et aux projets de recherche universitaire accélère encore la croissance. La demande croissante d'automatisation, d'optimisation des flux de travail et de soutien consultatif renforce la position des prestataires de services. Le marché est également soutenu par la sensibilisation croissante des cliniciens et des patients à la détection précoce des maladies et aux approches thérapeutiques personnalisées.
Part de marché des services de diagnostic moléculaire en Europe
L'industrie européenne des services de diagnostic moléculaire est principalement dirigée par des entreprises bien établies, notamment :
- F. Hoffmann-La Roche Ltd (États-Unis)
- QIAGEN (Allemagne)
- BIOMÉRIEUX (France)
- Illumina, Inc. (États-Unis)
- Thermo Fisher Scientific Inc. (États-Unis)
- Agilent Technologies, Inc. (États-Unis)
- Danaher Corporation (États-Unis)
- Sysmex Corporation (Japon)
- PerkinElmer (États-Unis)
- Hologic, Inc. (États-Unis)
- Fujirebio Europe NV (Belgique)
- Euroimmun Medizinische Labordiagnostika AG (Allemagne)
- Centogene AG (Allemagne)
- GeneProof sro (République tchèque)
- altona Diagnostics GmbH (Allemagne)
- Seegene Inc. (Corée du Sud)
- Aidian Oy (Finlande)
- Bioeksen Biotech (Turquie)
- LRE Medical GmbH (Allemagne)
Quels sont les développements récents sur le marché européen des services de diagnostic moléculaire ?
- En novembre 2024, Menarini Diagnostics et Nucleix ont conclu un accord commercial à long terme pour la distribution exclusive du test Bladder EpiCheck en Europe. Ce test urinaire non invasif, marqué CE, détecte les cancers primitifs ou récidivants de la vessie et des voies urinaires supérieures, et vise à transformer la prise en charge des patients atteints de cancer de la vessie en Europe.
- En octobre 2024, Seegene et Werfen ont finalisé un accord de partenariat pour le partage de technologies en diagnostic moléculaire. Cette collaboration vise à intégrer les technologies de diagnostic de Seegene aux systèmes d'automatisation de laboratoire de Werfen, afin d'améliorer l'efficacité diagnostique et d'étendre la portée des tests moléculaires en Europe.
- En octobre 2024, Alveo Technologies a commencé à expédier vers l'UE son premier test moléculaire ponctuel pour la grippe aviaire chez les volailles. Le panel Alveo Sense pour la grippe aviaire fournit des résultats rapides et précis sur le terrain, favorisant ainsi la détection précoce et les mesures de contrôle dans la filière avicole.
- En juillet 2024, Roche a annoncé l'acquisition de la technologie Point-of-Care de LumiraDx, enrichissant ainsi son portefeuille de diagnostics grâce à une plateforme multi-tests. Cette acquisition vise à élargir l'accès aux tests diagnostiques en médecine générale et soutient l'ambition de Roche de proposer des solutions décentralisées, notamment un test moléculaire rapide de dépistage de la tuberculose au point-of-care, en collaboration avec la Fondation Bill & Melinda Gates.
- En juillet 2024, AB ANALITICA et SNIBE ont annoncé un partenariat de distribution en Italie pour la plateforme Molecision R8 de SNIBE. Cette collaboration vise à améliorer la disponibilité de solutions de diagnostic moléculaire avancées sur le marché italien, en proposant aux professionnels de santé des technologies de test innovantes.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.