Marché européen des cellules souches pluripotentes induites (iPSC), par source cellulaire (cellules cutanées et cellules sanguines), type (IPSC humaines et IPSC de souris), produit (instruments, consommables et kits et services), applications (recherche universitaire, médecine régénérative, thérapie cellulaire, criblage toxicologique, découverte et développement de médicaments, modélisation des maladies, banque de cellules souches, bio-impression 3D et autres), utilisateur final (sociétés de biotechnologie et pharmaceutiques, laboratoires de recherche, laboratoires de diagnostic et autres), canal de distribution (appel d'offres direct et vente au détail), pays (Allemagne, Royaume-Uni, Italie, France, Espagne, Suisse, Russie, Turquie, Belgique, Pays-Bas et reste de l'Europe) - Tendances et prévisions de l'industrie jusqu'en 2029.
Analyse et perspectives du marché : marché européen des cellules souches pluripotentes induites (iPSC)
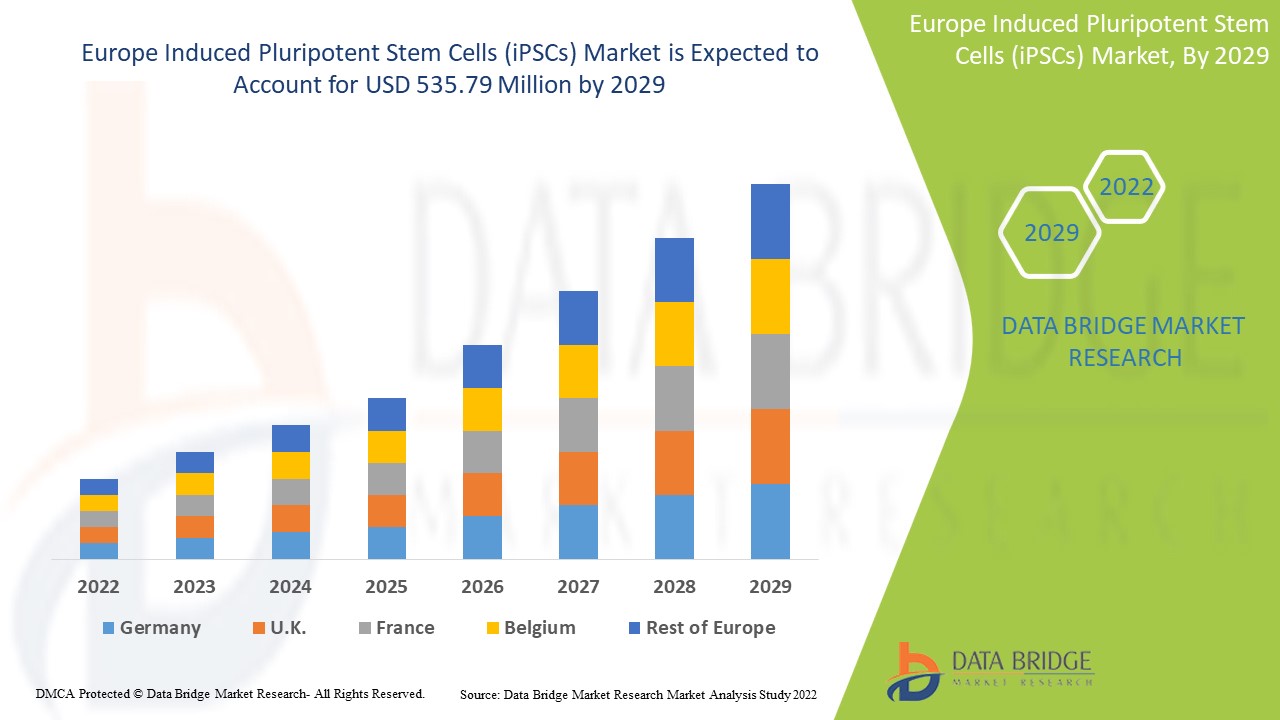
Le marché européen des cellules souches pluripotentes induites (iPSC) devrait connaître une croissance du marché au cours de la période de prévision de 2022 à 2029. Data Bridge Market Research analyse que le marché croît avec un TCAC de 8,9 % au cours de la période de prévision de 2022 à 2029 et devrait atteindre 535,79 millions USD d'ici 2029. L'augmentation des activités de recherche sur les thérapies à base de cellules souches agit comme moteur de la croissance du marché des cellules souches pluripotentes induites (iPSC).
Les cellules souches pluripotentes induites sont des types de cellules dérivées des tissus somatiques adultes et reprogrammées avec un ensemble de gènes et de facteurs pour obtenir une nature pluripotente. Certains gènes et facteurs sont ajoutés pour obtenir des propriétés définies des cellules souches embryonnaires. Les cellules pluripotentes induites étant presque identiques aux cellules donneuses, elles aident à la modélisation des maladies. Les rétrovirus sont couramment utilisés comme vecteurs pour reprogrammer les cellules souches pluripotentes induites. Les principales applications des cellules souches pluripotentes induites sont la modélisation des maladies, la découverte et le développement de médicaments, les études de toxicité et les thérapies géniques. Elles sont largement utilisées dans les traitements des maladies cardiovasculaires, du diabète sucré et de divers types de cancer. Les cellules souches pluripotentes induites humaines présentent les propriétés pertinentes de la maladie car elles portent le génotype spécifique de la maladie, permettant ainsi de nouvelles options thérapeutiques adaptées au patient.
L'adoption croissante de la thérapie par cellules souches, la croissance du secteur des biotechnologies avec de meilleurs investissements et la prévalence croissante des maladies chroniques sont les moteurs du marché des cellules souches pluripotentes induites (iPSC). Les autres facteurs qui devraient propulser la croissance du marché européen des cellules souches pluripotentes induites (iPSC) comprennent la large gamme d'applications cliniques des cellules souches pluripotentes induites et les avancées technologiques émergentes des iPSC.
Cependant, des facteurs tels que le coût élevé associé aux thérapies à base de cellules souches et la disponibilité d'alternatives pour le traitement des tumeurs freinent la croissance du marché européen des cellules souches pluripotentes induites (iPSC). D'autre part, le nombre croissant de produits en cours de développement, l'intérêt accru pour la médecine personnalisée et l'augmentation des dépenses de santé constituent une opportunité pour la croissance du marché européen des cellules souches pluripotentes induites (iPSC). Les règles et réglementations strictes et l'instabilité génomique des IPSC constituent le principal défi du marché européen des cellules souches pluripotentes induites (iPSC).
Le rapport sur le marché des cellules souches pluripotentes induites (iPSC) fournit des détails sur la part de marché, les nouveaux développements et l'analyse du pipeline de produits, l'impact des acteurs du marché national et local, analyse les opportunités en termes de poches de revenus émergentes, les changements dans la réglementation du marché, les approbations de produits, les décisions stratégiques, les lancements de produits, les expansions géographiques et les innovations technologiques sur le marché. Pour comprendre l'analyse et le scénario du marché des cellules souches pluripotentes induites (iPSC), contactez Data Bridge Market Research pour un briefing d'analyste, notre équipe vous aidera à créer une solution d'impact sur les revenus pour atteindre votre objectif souhaité.
Portée et taille du marché des cellules souches pluripotentes induites (iPSC)
Le marché des cellules souches pluripotentes induites (iPSC) est segmenté sur la base de la source cellulaire, du type, du produit, des applications, des utilisateurs finaux et du canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence dans vos marchés cibles.
Le marché européen des cellules souches pluripotentes induites (iPSC) est classé en six segments notables en fonction de la source cellulaire, du type, du produit, des applications, des utilisateurs finaux et du canal de distribution.
- En fonction de la source cellulaire, le marché européen des cellules souches pluripotentes induites (iPSC) est segmenté en cellules cutanées et en cellules sanguines. En 2022, le segment des cellules cutanées devrait dominer le marché en raison de la grande disponibilité des sources de cellules cutanées et de la forte notoriété de celles-ci.
- Sur la base du type, le marché européen des cellules souches pluripotentes induites (iPSC) est segmenté en cellules souches pluripotentes induites humaines et cellules souches pluripotentes induites de souris. En 2022, le segment des cellules souches pluripotentes induites humaines devrait dominer le marché en raison de l'expansion de la population gériatrique et du nombre croissant d'essais cliniques en France.
- Sur la base du produit, le marché européen des cellules souches pluripotentes induites (iPSC) est segmenté en instruments, consommables et kits et services. En 2022, le segment des consommables et kits devrait dominer le marché en raison de l'augmentation de la R&D au Royaume-Uni en raison de la présence d'experts en biologie du développement et de la reproduction.
- Sur la base des applications, le marché européen des cellules souches pluripotentes induites (iPSC) est segmenté en recherche universitaire, médecine régénérative , thérapie cellulaire, dépistage toxicologique, découverte et développement de médicaments, modélisation de maladies, banque de cellules souches, bio-impression 3D et autres. En 2022, le segment de la découverte et du développement de médicaments devrait dominer le marché, car l'externalisation des essais sur les iPSC a finalement renforcé le processus d'approbation des médicaments.
- Sur la base des utilisateurs finaux, le marché européen des cellules souches pluripotentes induites (iPSC) est segmenté en sociétés biotechnologiques et pharmaceutiques, laboratoires de recherche, laboratoires de diagnostic et autres. En 2022, le segment des sociétés biotechnologiques et pharmaceutiques devrait dominer le marché, car la région est récemment devenue la plaque tournante des petites et grandes entreprises biopharmaceutiques et est devenue dépendante du secteur des organismes de recherche sous contrat et d'autres services cliniques pour les opérations de recherche et développement.
- Sur la base du canal de distribution, le marché européen des cellules souches pluripotentes induites (iPSC) est segmenté en ventes directes et ventes au détail. En 2022, le segment des ventes directes devrait dominer le marché en raison du nombre élevé de sources de distribution.
Analyse du marché des cellules souches pluripotentes induites (iPSC) au niveau des pays
Le marché des cellules souches pluripotentes induites (iPSC) est analysé et des informations sur la taille du marché sont fournies par pays, source de cellules, type, produit, applications, utilisateurs finaux et canal de distribution comme référencé ci-dessus.
Les pays couverts par le rapport sur le marché des cellules souches pluripotentes induites (iPSC) sont le Royaume-Uni, l’Allemagne, la France, l’Espagne, l’Italie, les Pays-Bas, la Suisse, la Russie, la Turquie, l’Autriche, l’Irlande et le reste de l’Europe.
Le segment des cellules souches embryonnaires humaines (iPSC) en Allemagne devrait connaître le taux de croissance le plus élevé au cours de la période de prévision de 2022 à 2029 en raison de l'utilisation croissante de la technologie des cellules souches. Le segment des cellules souches embryonnaires humaines (iPSC) en France domine le marché européen en raison de l'augmentation des cas de maladies chroniques et de l'adoption massive de sources de cellules souches pour de meilleures thérapies. Le Royaume-Uni est en tête de la croissance du marché et le segment des cellules souches embryonnaires humaines domine dans ce pays en raison du nombre croissant de centres de biotechnologie et d'activités de recherche.
La section pays du rapport fournit également des facteurs d'impact sur les marchés individuels et des changements de réglementation sur le marché national qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie des pays, les actes réglementaires et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques européennes et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des canaux de vente sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Growing Strategic Activities by Major Market Players to Enhance the Awareness for Induced Pluripotent Stem Cells (iPSCs) Treatment, is Boosting the Market Growth of Induced Pluripotent Stem Cells (iPSCs) Market.
The induced pluripotent stem cells (iPSCs) market also provides you with detailed market analysis for every country growth in particular market. Additionally, it provides the detail information regarding the market players’ strategy and their geographical presence. The data is available for historic period 2011 to 2020.
Competitive Landscape and Induced Pluripotent Stem Cells (iPSCs) Market Share Analysis
Induced pluripotent stem cells (iPSCs) market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to induce pluripotent stem cells (iPSCs) market.
The major companies which are dealing in the induced pluripotent stem cells (iPSCs) are Thermo Fisher Scientific Inc., FUJIFILM Corporation, LumaCyte, Horizon Discovery Ltd., Takara Bio Inc., Lonza., Evotec SE., Axol Bioscience Ltd., R & D Systems, Inc., Charles River Laboratories International, Inc., Corning Incorporated, REPROCELL Inc., Merck KGaA and among others other domestic players. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Many contract and agreement are also initiated by the companies’ worldwide which are also accelerating the induced pluripotent stem cells (iPSCs) market.
For instance,
- In February 2021, Thermo Fisher Scientific Inc. announced it has won six awards in the annual CMO Leadership Awards. The awards, presented by Life Science Leader and Outsourced Pharma, recognize top contract manufacturing partners as evaluated by biopharma and biotech companies. It is estimated that this recognition anticipated to strengthen company’s footprints in Europe market and leading to upsurge Company’s growth in the coming years.
- In June 2020, the LumaCyte collaborated with Catalent who are the Europe provider of advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, cell and gene therapies, and consumer health products. This collaboration aided in expanding the company’s stem cell technology product Radiance and its application.
Collaboration, product launch, business expansion, award and recognition, joint ventures and other strategies by the market player is enhancing the company footprints in the veterinary infusion pumps market which also provides the benefit for organization’s profit growth.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CELL SOURCE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 EUROPE MEDICAL CARTS MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.2 REGULATION IN CANADA
5.3 REGULATION IN EUROPE
5.4 REGULATION IN INDIA
5.5 REGUALTION IN JAPAN
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 WIDE RANGE OF CLINICAL APPLICATION OF INDUCED PLURIPOTENT STEM CELLS
6.1.2 EMERGING TECHNOLOGICAL ADVANTAGES OF IPSCS
6.1.3 RISING PREVALENCE OF SEVERAL CHRONIC DISEASES
6.1.4 INCREASING ADOPTION OF STEM CELL THERAPY
6.1.5 GROWING BIOTECHNOLOGY SECTOR WITH BETTER INVESTMENT
6.2 RESTRAINT
6.2.1 HIGH COST ASSOCIATED WITH STEM CELL THERAPIES AND LARGE-SCALE APPLICATIONS OF IPSCS
6.2.2 AVAILABILITY OF ALTERNATIVES FOR TUMOR TREATMENT
6.2.3 ADVERSE EFFECTS OF STEM CELL TRANSPLANTS
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE PRODUCTS
6.3.2 INCREASING INTEREST OF PERSONALIZED MEDICINE
6.3.3 SURGE IN HEALTHCARE EXPENDITURE
6.3.4 STRATEGIC INITIATIVES BY KEY MARKET PLAYERS
6.4 CHALLENGES
6.4.1 GENOMIC INSTABILITY OF IPSCS IS THE KEY MARKET CHALLENGE
6.4.2 LACK OF SKILLED PROFESSIONALS
6.4.3 STRINGENT REGULATORY FRAMEWORK
7 IMPACT OF COVID-19 ON THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
7.1 IMPACT ON PRICE
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY CHAIN
7.4 STRATEGIC DECISIONS BY MANUFACTURERS
7.5 CONCLUSION
8 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE
8.1 OVERVIEW
8.2 SKIN CELLS
8.2.1 FIBROBLAST
8.2.2 KERATINOCYTES
8.2.3 ADIPOSE DERIVED STEM CELLS
8.2.4 HEPATOCYTES
8.2.5 MELANOCYTES
8.2.6 NEURAL STEM CELLS
8.2.7 OTHERS
8.3 BLOOD CELLS
8.3.1 PERIPHERAL BLOOD
8.3.2 CORD BLOOD ENDOTHELIAL CELLS
8.3.3 CORD BLOOD STEM CELLS
8.3.4 OTHERS
9 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE
9.1 OVERVIEW
9.2 HUMAN IPSCS
9.3 MOUSE IPSCS
10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT
10.1 OVERVIEW
10.2 CONSUMABLES & KITS
10.2.1 REPROGRAMMING KITS
10.2.2 MEDIA
10.2.3 TRANSFECTION KITS
10.2.4 CELL IDENTIFICATION KITS
10.2.5 ACCESSORIES
10.2.6 OTHERS
10.3 SERVICES
10.4 INSTRUMENTS
10.4.1 IMAGING SYSTEMS
10.4.2 ELECTROPORATION DEVICE
10.4.3 INCUBATORS
10.4.4 OTHERS
11 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 DRUG DISCOVERY AND DEVELOPMENT
11.3 ACADEMIC RESEARCH
11.4 DISEASE MODELLING
11.5 CELLULAR THERAPY
11.6 REGENERATIVE MEDICINE
11.7 TOXICOLOGY SCREENING
11.8 STEM CELL BANKING
11.9 3D BIOPRINTING
11.1 OTHERS
12 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER
12.1 OVERVIEW
12.2 BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES
12.3 RESEARCH LABORATORIES
12.4 DIAGNOSTIC LABORATORIES
12.5 OTHERS
13 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
14 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 FRANCE
14.1.3 U.K.
14.1.4 ITALY
14.1.5 RUSSIA
14.1.6 SPAIN
14.1.7 TURKEY
14.1.8 NETHERLANDS
14.1.9 SWITZERLAND
14.1.10 BELGIUM
14.1.11 REST OF EUROPE
15 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 FUJIFILM CORPORATION
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.1.5.1 ACQUISITION
17.2 THERMO FISHER SCIENTIFIC INC.
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.2.5.1 EVENT
17.2.5.2 ACQUISITION
17.3 LONZA.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.3.5.1 EXPANSION
17.4 MERCK KGAA
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.4.5.1 AGREEMENT
17.5 EVOTEC SE.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENTS
17.5.5.1 AGREEMENT
17.5.5.2 COLLABORATION
17.6 APPLIED STEMCELL.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENTS
17.6.3.1 PRODUCT LAUNCH
17.7 AXOL BIOSCIENCE LTD.
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.7.3.1 MERGER
17.7.3.2 PRODUCT LAUNCH
17.8 CELL APPLICATIONS, INC.
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.8.3.1 PARTNERSHIP
17.9 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENT
17.9.4.1 ACQUISITION
17.1 CITIUS PHARMACEUTICALS, INC.
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.10.3.1 AGREEMENT
17.11 CORNING INCORPORATED
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.11.4.1 AGREEMENT
17.12 FATE THERAPEUTICS
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENT
17.12.3.1 CLINICAL TRIAL
17.13 GENECOPOEIA, INC.
17.13.1 COMPANY SNAPSHOT
17.13.2 PRODUCT PORTFOLIO
17.13.3 RECENT DEVELOPMENT
17.14 HOPSTEM BIOTECHNOLOGY LLC.
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.14.3.1 PARTNERSHIP
17.15 HORIZON DISCOVERY LTD.
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENT
17.16 LUMACYTE
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENT
17.16.3.1 COLLABORATION
17.17 R & D SYSTEMS, INC.
17.17.1 COMPANY SNAPSHOT
17.17.2 REVENUE ANALYSIS
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENT
17.18 REPROCELL INC.
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENTS
17.18.3.1 COLLABORATION
17.18.3.2 FACILITY EXPANSION
17.18.3.3 SERVICE LAUNCH
17.19 TAKARA BIO INC.
17.19.1 COMPANY SNAPSHOT
17.19.2 REVENUE ANALYSIS
17.19.3 PRODUCT PORTFOLIO
17.19.4 RECENT DEVELOPMENTS
17.19.4.1 NEW FACILITY LAUNCH
17.2 UNIVERSAL CELLS INC. (AN ASTELLAS COMPANY)
17.20.1 COMPANY SNAPSHOT
17.20.2 REVENUE ANALYSIS
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENT
17.20.4.1 ACQUISITION
18 QUESTIONNAIRE
19 RELATED REPORTS
Liste des tableaux
TABLE 1 NEW CANCER CASES, AGES 85+, IN THE U.S.
TABLE 2 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 3 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 7 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 EUROPE HUMAN IPSCS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE MOUSE IPSCS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 11 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 13 EUROPE SERVICES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 16 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE DRUG DISCOVERY AND DEVELOPMENT IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ACADEMIC RESEARCH IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE DISEASE MODELLING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE CELLULAR THERAPY IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE REGENERATIVE MEDICINE IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE TOXICOLOGY SCREENING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE STEM CELL BANKING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE 3D BIOPRINTING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE OTHERS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 27 EUROPE BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE RESEARCH LABORATORIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE DIAGNOSTIC LABORATORIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE OTHERS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 32 EUROPE DIRECT TENDER IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE RETAIL SALES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 35 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 36 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 37 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 38 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 39 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 40 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 41 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 42 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 46 GERMANY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 47 GERMANY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 50 GERMANY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 51 GERMANY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 52 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 53 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 54 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 55 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 56 FRANCE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 57 FRANCE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 58 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 59 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 60 FRANCE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 61 FRANCE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 62 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 65 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 66 U.K. SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 67 U.K. BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 68 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 69 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 70 U.K. INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 71 U.K. CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 72 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 73 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 76 ITALY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 77 ITALY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 78 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 ITALY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 81 ITALY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 82 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 84 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 88 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 89 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 90 RUSSIA INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 91 RUSSIA CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 92 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 93 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 95 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 96 SPAIN SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 97 SPAIN BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 98 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 99 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 100 SPAIN INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 101 SPAIN CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 103 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 104 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 105 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 106 TURKEY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 107 TURKEY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 108 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 109 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 110 TURKEY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 111 TURKEY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 112 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 114 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 115 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 116 NETHERLANDS SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 117 NETHERLANDS BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 118 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 119 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 120 NETHERLANDS INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 NETHERLANDS CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 122 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 123 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 124 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 125 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 126 SWITZERLAND SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 127 SWITZERLAND BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 128 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 129 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 130 SWITZERLAND INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 131 SWITZERLAND CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 132 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 133 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 134 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 135 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 136 BELGIUM SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 137 BELGIUM BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 138 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 139 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 140 BELGIUM INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 141 BELGIUM CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 142 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 143 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 144 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 145 REST OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SEGMENTATION
FIGURE 2 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SEGMENTATION
FIGURE 11 THE WIDE RANGE OF CLINICAL APPLICATION OF INDUCED PLURIPOTENT STEM CELLS (IPSC) ARE EXPECTED TO DRIVE THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SKIN CELLS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
FIGURE 15 PREVALENCE OF CHRONIC DISEASES
FIGURE 16 NUMBER OF PEOPLE WITH DIABETES (MILLION) AMONG AGES 20–79 YEARS
FIGURE 17 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, 2021
FIGURE 18 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, 2020-2029 (USD MILLION)
FIGURE 19 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, CAGR (2022-2029)
FIGURE 20 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, LIFELINE CURVE
FIGURE 21 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, 2021
FIGURE 22 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, 2020-2029 (USD MILLION)
FIGURE 23 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 24 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, 2021
FIGURE 26 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, 2020-2029 (USD MILLION)
FIGURE 27 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 28 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 29 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, 2021
FIGURE 30 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, 2020-2029 (USD MILLION)
FIGURE 31 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 32 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 33 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, 2021
FIGURE 34 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 35 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, CAGR (2022-2029)
FIGURE 36 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 38 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 39 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 40 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SNAPSHOT (2021)
FIGURE 42 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2021)
FIGURE 43 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 44 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 45 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE (2022-2029)
FIGURE 46 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.