Asia Pacific Chinese Hamster Ovary Cells Cho Market
Taille du marché en milliards USD
TCAC :
% 
 USD
87.85 Million
USD
188.52 Million
2024
2032
USD
87.85 Million
USD
188.52 Million
2024
2032
| 2025 –2032 | |
| USD 87.85 Million | |
| USD 188.52 Million | |
|
|
|
|
Segmentation des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique : type (services et produits), système (système de sélection métabolique, système de sélection antibiotique et autres), application (produits biologiques et recherche médicale), utilisateur final (entreprises biopharmaceutiques, entreprises de biotechnologie, organismes de développement clinique et de fabrication, organismes de recherche clinique, instituts et organismes de recherche universitaires et autres), canal de distribution (appels d’offres directs, vente au détail et autres) – Tendances du secteur et prévisions jusqu’en 2032
Taille du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique
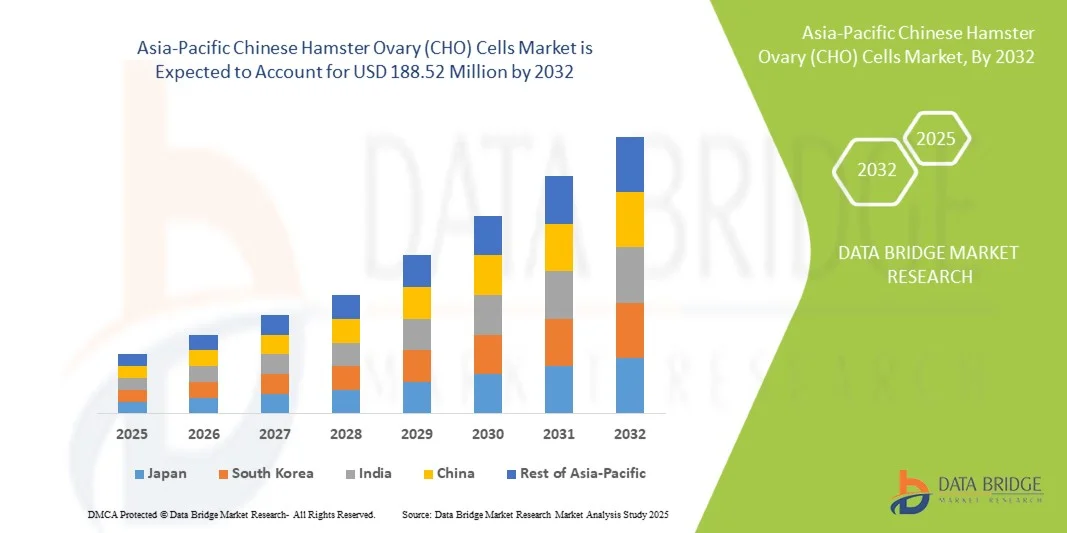
- Le marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique était évalué à 87,85 millions de dollars américains en 2024 et devrait atteindre 188,52 millions de dollars américains d'ici 2032, avec un TCAC de 10,1 % au cours de la période de prévision.
- Cette croissance est alimentée par des facteurs tels que la demande croissante de produits biologiques, les progrès en ingénierie des lignées cellulaires, l'augmentation des investissements dans la recherche en biotechnologie et le développement des applications de la thérapie génique.
- De plus, les progrès technologiques constants en matière d'ingénierie des lignées cellulaires, d'optimisation des procédés et d'automatisation de la bioproduction améliorent la productivité et la qualité des produits. La convergence de ces facteurs accélère l'utilisation des cellules CHO dans le développement des produits biologiques, stimulant ainsi considérablement la croissance du marché.
Analyse du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique
- Les cellules CHO (ovaires de hamster chinois) sont une lignée cellulaire de mammifères couramment utilisée en biotechnologie et en recherche biomédicale, notamment pour la culture cellulaire et les bioprocédés. Elles sont devenues des outils essentiels dans ces domaines. Les cellules CHO sont reconnues pour leur adhérence aux supports de culture, leur croissance rapide et leur capacité à exprimer des protéines recombinantes.
- L'une des principales applications des cellules CHO réside dans la production de protéines recombinantes, notamment d'anticorps thérapeutiques et d'enzymes. Elles peuvent être génétiquement modifiées pour exprimer des gènes d'intérêt spécifiques, et leur capacité à effectuer des modifications post-traductionnelles similaires à celles des cellules humaines garantit la qualité des produits biopharmaceutiques. Les cellules CHO sont également reconnues pour leur stabilité génétique, ce qui leur permet d'introduire des modifications génétiques de manière constante sur plusieurs générations, assurant ainsi la fiabilité et la reproductibilité des bioprocédés.
- En 2025, la Chine devrait dominer le marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique avec une part de marché de 24,04 %, tandis que le Japon devrait être la région à la croissance la plus rapide avec un TCAC de 10,5 % en raison de l'augmentation de la recherche biopharmaceutique, de l'expansion des capacités de fabrication de produits biologiques, de la hausse des investissements dans la biotechnologie et des initiatives gouvernementales de soutien promouvant la production de biosimilaires.
- En 2025, le segment des services devrait dominer le marché avec une part de marché de 67,74 % en raison de la demande croissante de services spécialisés liés au développement de lignées cellulaires CHO, au bioprocédé et à la fabrication à façon.
Portée du rapport et segmentation du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique
|
Attributs |
Cellules ovariennes de hamster chinois (CHO) : principaux enseignements du marché |
|
Segments couverts |
|
|
Pays couverts |
Asie-Pacifique
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur du marché, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché élaborés par Data Bridge Market Research comprennent également une analyse des importations et des exportations, un aperçu de la capacité de production, une analyse de la consommation de production, une analyse des tendances des prix, un scénario de changement climatique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, les critères de sélection des fournisseurs, une analyse PESTLE, une analyse de Porter et le cadre réglementaire. |
Tendances du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique
« Intégration croissante de l’intelligence artificielle (IA) dans le développement des lignées cellulaires »
- L'une des tendances majeures du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique est l'intégration croissante de l'intelligence artificielle (IA) dans le développement des lignées cellulaires et l'optimisation des bioprocédés.
- Les algorithmes d'IA sont utilisés pour analyser de grands ensembles de données, prédire les conditions de culture optimales et identifier les clones à haut rendement plus efficacement que les méthodes traditionnelles.
- Par exemple, les modèles d'apprentissage automatique peuvent traiter des données expérimentales pour prédire les performances cellulaires, réduisant ainsi le temps et les coûts associés aux approches par essais et erreurs dans la production à base de cellules CHO.
- Cette technologie permet des délais de développement plus rapides, une meilleure qualité des produits et une meilleure évolutivité de la production de produits biologiques.
- L'utilisation de l'IA transforme la recherche sur les cellules CHO et la bioproduction, ce qui se traduit par une efficacité accrue, des coûts de développement réduits et un avantage concurrentiel pour les entreprises qui adoptent des solutions numériques avancées.
Dynamique du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique
Conducteur
« Utilisation croissante des cellules CHO dans les études génétiques »
- L'utilisation croissante des cellules ovariennes de hamster chinois (CHO) en recherche génétique est un moteur de croissance important et en pleine expansion pour le marché des CHO en Asie-Pacifique. Avec la maturation des outils génétiques (CRISPR, RMCE, RNA-seq, multi-omique unicellulaire), les cellules CHO ne sont plus de simples hôtes de production ; elles constituent désormais des plateformes expérimentales permettant d'étudier les relations génotype-phénotype, de tester des stratégies d'édition génique et de concevoir rationnellement des caractéristiques de l'hôte (croissance, productivité, glycosylation, tolérance au stress).
- Cette évolution accroît la demande sur trois axes de marché interdépendants : (1) en amont – lignées cellulaires CHO sous licence/modifiées et services d’édition génique ; (2) développement de procédés – outils d’analyse, d’omique, de jumeau numérique et d’IA nécessaires pour traduire les connaissances génétiques en clones stables à haut rendement ; et (3) fabrication – capacité des CDMO et plateformes à usage unique/continues optimisées pour les hôtes génétiquement améliorés.
- En bref, les études génétiques alimentent un écosystème en expansion (logiciels, analyses, banques de cellules, réactifs, CDMO), augmentant ainsi l'étendue et la profondeur des offres commerciales de cellules CHO et, par conséquent, la taille du marché, le chiffre d'affaires et l'importance stratégique des capacités de production dérivées de cellules CHO.
- Par exemple, en janvier 2025, un article du NIH indiquait qu'un jeu de données de criblage par inactivation génique CRISPR non viral à l'échelle du génome pour les cellules CHO-K1 et les cellules recombinantes dérivées avait été publié (Sci Data), fournissant une ressource complète pour identifier les gènes qui affectent la viabilité cellulaire et la production de protéines – preuve que le criblage génomique fonctionnel dans les cellules CHO est désormais mature, documenté publiquement et contribue directement aux programmes d'ingénierie cellulaire.
- En avril 2025, un article publié dans NIH indiquait qu'une méta-analyse des transcriptomes de cellules CHO (« Fantastic genes… ») avait été acceptée et publiée dans PMC (en accès libre) après révision début 2025. Cet article intègre des données de séquençage d'ARN et épigénétiques issues de centaines d'échantillons de cellules CHO et souligne la nécessité d'une ingénierie génétique ciblée pour contrôler les programmes d'expression. Ceci constitue une preuve directe que les études génomiques et épigénomiques des cellules CHO définissent des cibles d'ingénierie pour l'amélioration des lignées cellulaires industrielles.
- L'utilisation croissante des cellules CHO dans les études génétiques renforce non seulement leur position dominante en tant que système d'expression de référence pour les produits biologiques, mais étend également leur rôle en tant que plateformes expérimentales pour la génomique fonctionnelle, les tests réglementaires et l'ingénierie cellulaire.
Retenue/Défi
« Le coût élevé de la production à base de cellules CHO constitue un frein au marché »
- Les coûts d'investissement et d'exploitation élevés associés à la production de produits biologiques à base de cellules CHO constituent un frein majeur au marché des cellules CHO en Asie-Pacifique.
- Les investissements initiaux importants dans la capacité des bioréacteurs, les salles blanches, les systèmes à usage unique ou l'infrastructure en acier inoxydable, et les équipements de purification spécialisés en aval — combinés à des matières premières coûteuses (milieux de culture, résines de chromatographie), à une main-d'œuvre qualifiée, à une conformité réglementaire complexe et à de longs délais de validation — augmentent le coût total de possession pour les fabricants et les CDMO.
- Ces pressions sur les coûts allongent les délais de commercialisation, compriment les marges, dissuadent les petits développeurs de produire en interne et peuvent ralentir l'adoption dans les régions à faibles revenus ; collectivement, elles limitent la demande de nouvelles lignées cellulaires, de consommables et de contrats de service liés aux plateformes CHO
- Par exemple, en juillet 2025, le Bureau du secrétaire adjoint à la planification et à l'évaluation du département américain de la Santé et des Services sociaux (HHS-ASPE) a publié une note d'information soulignant que les dépenses consacrées aux produits biologiques ont considérablement augmenté et que leur complexité et leur coût (développement et fabrication) sont des facteurs importants de cette hausse, mettant ainsi en évidence les contraintes économiques que représentent les coûts de fabrication des produits biologiques et des CHO.
- En mars 2024, la Commission européenne a publié des documents d'orientation et de recherche (analyses du Centre commun de recherche / DG R&I) soulignant que les biotechnologies et la bioproduction nécessitent des équipements hautement spécialisés et une main-d'œuvre multidisciplinaire qualifiée – des facteurs qui augmentent les coûts unitaires de production et constituent des obstacles à l'industrialisation à grande échelle dans l'UE.
- Bien que la production de protéines thérapeutiques et d'anticorps monoclonaux par culture cellulaire CHO soit la méthode de référence, son développement reste fortement limité par des coûts élevés. Les infrastructures coûteuses, les matières premières onéreuses, la main-d'œuvre spécialisée requise et les procédures de conformité rigoureuses alourdissent les dépenses de production, limitant la participation des petites entreprises et entravant l'accès aux marchés émergents.
Portée du marché des cellules ovariennes de hamster chinois (CHO) en Asie-Pacifique
Le marché est segmenté en fonction du type, du système, de l'application, de l'utilisateur final et du canal de distribution.
- Par type
On the basis of type, the market is segmented into services, products and others, In 2025, the services segment is expected to dominate the market with 67.74%, and expected to be fastest growing segment with 10.3% CAGR. This growth can be attributed to the increasing reliance on contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) for specialized cell line development, process optimization, and large-scale biomanufacturing. Additionally, the rising complexity of biologics production, high costs associated with in-house facilities, and growing demand for customized solutions are encouraging pharmaceutical and biotechnology companies to outsource more services. These factors collectively drive the expansion of the services segment, enhancing its market share and growth potential over the forecast period.
- By System
On the basis of system, the market is segmented into Metabolic Selection System, Antibiotic Selection System, and Others, In 2025, the Metabolic Selection System segment is expected to dominate the market with 60.88%, and expected to be fastest growing segment with 10.3% CAGR, This growth is primarily driven by the increasing adoption of metabolic selection systems for stable and high-yield recombinant protein production, as they eliminate the need for antibiotic resistance markers, reduce regulatory concerns, and enhance culture efficiency. Moreover, the growing preference for safer, cost-effective, and scalable cell line development techniques in biologics and biosimilar manufacturing further supports the expansion of this segment.
- By Application
On the basis of application, the market is segmented into Biologics, and Medical Research, In 2025, the Biologics segment is expected to dominate the market with 73.32% and expected to be fastest growing segment with 10.22% CAGR, This growth is mainly attributed to the increasing demand for therapeutic proteins, monoclonal antibodies, and vaccines, coupled with the rising prevalence of chronic and autoimmune diseases. Additionally, advancements in bioprocessing technologies, expanding biopharmaceutical R&D investments, and the growing adoption of Chinese Hamster Ovary (CHO) cells for high-quality and scalable biologics production further propel the dominance and rapid expansion of the Biologics segment in the market.
- By End User
On the basis of end user, the market is segmented into Biopharmaceutical Companies, Biotechnology Companies, Clinical Development and Manufacturing Organizations, Clinical Research Organizations, Academic Institutes and Research Organizations, and Others, In 2025, the Biopharmaceutical Companies segment is expected to dominate the market with 46.80%, This dominance is driven by the increasing focus of biopharmaceutical companies on developing biologics, monoclonal antibodies, and gene therapies, which heavily rely on advanced cell lines like CHO cells for high-yield and high-quality protein production. Additionally, rising R&D investments, the expansion of biologics pipelines, and the need for scalable and cost-effective production processes further reinforce the leading position of biopharmaceutical companies in the market.
- By Distribution Channel
On the basis of Distribution Channel, the market is segmented into Direct Tenders, Retail Sales, and Others, In 2025, the Direct Tenders segment is expected to dominate the market with 58.36%, and Retail Sales expected to be fastest growing segment with 9.9% CAGR. The dominance of direct tenders is driven by bulk procurement by large biopharmaceutical and research organizations seeking cost efficiency, reliable supply, and long-term contracts. Meanwhile, the rapid growth of retail sales is fueled by increasing accessibility of research products to smaller laboratories and academic institutes, rising demand for ready-to-use kits, and the expansion of e-commerce platforms for scientific supplies, making products more readily available across diverse end users.
Asia-Pacific Chinese Hamster Ovary (CHO) Cells Market Regional Analysis
- Asia-Pacific holds the share of 22.33% in the global CHO cells market, driven by a well-established biopharmaceutical industry, advanced healthcare infrastructure, and the strong presence of leading pharmaceutical and biotechnology companies.
- The United States, in particular, contributes significantly to regional dominance due to high R&D spending, increasing demand for biologics, and extensive use of CHO cells in the production of therapeutic proteins such as monoclonal antibodies.
- Government support for biologics development, coupled with robust regulatory frameworks and favorable reimbursement policies, further accelerates market growth. Moreover, strategic collaborations, technological advancements in cell line development, and an increase in FDA approvals for CHO cell-derived products continue to boost market expansion across the region.
China CHO cells Market Insight
- The China CHO cells market accounted for the largest market revenue share in the Asia-Pacific CHO cells market in 2025, attributed to the country’s well-established biopharmaceutical industry, significant R&D investments, presence of major biotechnology and pharmaceutical companies, advanced biologics manufacturing infrastructure, and strong government support for innovation in cell line development and biologics production.
Japan CHO cells Market Insight
- The Japan CHO cells market is expected to register a significant CAGR in Asia-Pacific from 2025 to 2032, driven by growing investments in biopharmaceutical research, increasing adoption of advanced cell line technologies, expansion of biologics and biosimilar production facilities, and supportive government initiatives promoting biotechnology and life sciences innovation.
Asia-Pacific Chinese Hamster Ovary (CHO) Cells Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific Inc. (U.S.)
- AcceGen (U.S.)
- RayBiotech Life, Inc. (U.S.)
- Cytion (Germany)
- BPS Bioscience, Inc. (U.S.)
- GenTarget Inc. (U.S.)
- Merck KGaA (Germany)
- Promega Corporation (U.S.)
- Abeomics (U.S.)
- Applied Biological Materials Inc. (Canada)
- ATCC (U.S.)
- Sartorius AG (Germany)
- Lonza (Switzerland)
- Revvity Discovery Limited (U.S.)
- Cytiva (U.S.)
- GTP Bioways (France)
- Curia Global, Inc. (U.S.)
- Abbott (U.S.)
Latest Developments in Asia-Pacific Chinese Hamster Ovary (CHO) Cells
- In February 2022, Sartorius acquired business from Novasep, added a complementary offering to its chromatography portfolio. The acquired portfolio includes chromatography systems primarily suited for small biomolecules such as oligonucleotides, peptides, and insulin, and innovative systems for the continuous production of biopharmaceuticals.
- In July 2023, Lonza launched TheraPRO CHO Media System, a new cell culture medium that simplifies processes and optimizes productivity and protein quality when using GS-CHO cell lines. The start-up supports pharmaceutical and biotech companies producing therapeutic proteins to further improve product quality. The TheraPRO CHO Media System provides efficient performance, achieving high concentrations of viable cells and protein titers above 5 g/L over a 15-day culture period. This represents more than double the protein titer that can be produced with commercially available solutions. This launch has helped the company to expand its product portfolio in the market.
- In October 2022, Thermo Fisher Scientific Inc. collaborated with ProBioGen to develop better platform the Gibco Freedom ExpiCHO-S Cell Line Development Kit. This kit allows users to generate cell lines suitable for clinical development without their own original cells, vectors, or previous experience in the field. ProBioGen significantly contributed to the performance of the Freedom ExpiCHO-S kit by leveraging its strong expertise in cell line and process development. The new series utilizes Thermo Fisher's ExpiCHO-S cell line expanding the company's product portfolio for the CHO cell line development series.
- In July 2023, Merck announced that it is expanding its facility in Lenexa, Kansas, U.S., adding 9,100 square meters of laboratory space and production capacity for the production of cell culture media. This expansion makes Lenexa the company's largest dry powder cell culture facility and a center of excellence in North America. The investment in the region reflects the company's strategy to expand and diversify its supply chain to meet current and future demand for cell culture platforms.
- In November 2022, ATCC, the world's leading regulatory and standards organization for biological materials, announced a new line of CAR-T Target luciferase reporter cell lines to support immuno-oncology (IO) discovery and the development of new immunotherapies. These models have a high endogenous expression of relevant chimeric antigen receptor (CAR) T target antigens such as HER2, CD19, and CD20. These new IO tools consist of both hematologic cancers and solid tumor cell lines expressing a luciferase reporter. This helped the company to expand its product portfolio.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTER'S FIVE FORCES ANALYSIS
4.3 PATENT ANALYSIS – ASIA-PACIFIC CHO CELLS MARKET
4.3.1 PATENT QUALITY AND STRENGTH
4.3.2 PATENT FAMILIES
4.3.3 LICENSING AND COLLABORATIONS
4.3.4 COMPETITIVE LANDSCAPE
4.3.5 IP STRATEGY AND MANAGEMENT
4.3.6 OTHER OBSERVATIONS
4.4 INDUSTRY INSIGHTS
4.4.1 MICRO AND MACRO ECONOMIC FACTORS
4.4.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.4.3 KEY PRICING STRATEGIES
4.5 INNOVATION TRACKER AND STRATEGIC ANALYSIS
4.5.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
4.5.1.1 JOINT VENTURES
4.5.1.2 MERGERS AND ACQUISITIONS
4.5.1.3 LICENSING AND PARTNERSHIP
4.5.1.4 TECHNOLOGY COLLABORATIONS
4.5.1.5 STRATEGIC DIVESTMENTS
4.5.2 NUMBER OF PRODUCTS IN DEVELOPMENT
4.5.3 STAGE OF DEVELOPMENT
4.5.4 TIMELINES AND MILESTONES
4.5.5 INNOVATION STRATEGIES AND METHODOLOGIES
4.5.6 RISK ASSESSMENT AND MITIGATION
4.5.7 FUTURE OUTLOOK
4.6 OPPORTUNITY MAP
4.7 PRICING ANALYSIS – ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET
4.8 RAW MATERIAL COVERAGE
4.9 VALUE CHAIN ANALYSIS
4.1 CONSUMER BUYING BEHAVIOUR
4.11 TECHNOLOGICAL ADVANCEMENTS
5 TARIFFS & IMPACT ON THE MARKET
5.1 CURRENT TARIFF RATE
5.2 OUTLOOK — LOCAL PRODUCTION VS. IMPORT RELIANCE
5.3 VENDOR SELECTION CRITERIA DYNAMICS
5.4 IMPACT ON SUPPLY CHAIN
5.4.1 RAW MATERIAL PROCUREMENT
5.4.2 MANUFACTURING AND PRODUCTION
5.4.3 LOGISTICS AND DISTRIBUTION
5.4.4 PRICE PITCHING AND MARKET POSITION
5.5 INDUSTRY PARTICIPANTS
5.5.1 SUPPLY CHAIN OPTIMIZATION
5.5.2 JOINT VENTURE AND LOCAL PARTNERSHIPS
5.6 IMPACT ON PRICES
5.7 REGULATORY INCLINATION
5.7.1 GEOPOLITICAL SITUATION
5.7.2 TRADE PARTNERSHIPS BETWEEN COUNTRIES
5.7.2.1 FREE TRADE AGREEMENTS
5.7.2.2 ALLIANCES ESTABLISHMENTS
5.7.3 STATUS ACCREDITATION (INCLUDING MFN)
5.7.4 DOMESTIC COURSE OF CORRECTION
5.7.4.1 INCENTIVE SCHEMES TO BOOST PRODUCTION OUTPUTS
5.7.4.2 ESTABLISHMENT OF SPECIAL ECONOMIC ZONES / INDUSTRIAL PARKS
6 REGULATORY FRAMEWORK – ASIA-PACIFIC CHO CELLS MARKET
6.1 NORTH AMERICA
6.2 EUROPE
6.3 ASIA-PACIFIC
6.4 SOUTH AMERICA
6.5 MIDDLE EAST & AFRICA
7 MARKET OVERVIEW
7.1 DRIVER
7.1.1 RISING USE OF CHO CELLS IN THE GENETIC STUDY
7.1.2 GROWING DEMAND FOR BIOPHARMACEUTICALS
7.1.3 RISING INVESTMENTS IN BIOTECHNOLOGY R&D
7.1.4 RISING DEMAND FOR MONOCLONAL ANTIBODIES
7.2 RESTRAINT
7.2.1 HIGH COST OF CHO CELL–BASED PRODUCTION AS A MARKET RESTRAINT
7.2.2 STRICT REGULATORY REQUIREMENTS FOR CHO CELL-BASED PRODUCTION
7.3 OPPORTUNITY
7.3.1 CONTINUOUS DEVELOPMENT OF CELL-CULTURE TECHNOLOGIES
7.3.2 RISING NUMBER OF APPLICATIONS OF CHO CELLS
7.3.3 ADVANCES IN CELL-LINE ENGINEERING & SYNTHETIC BIOLOGY
7.4 CHALLENGES
7.4.1 TIME-CONSUMING AND INCONSISTENCY IN CHO CELL LINE DEVELOPMENT PROCESS
7.4.2 CONTAMINATION RISK OF CHO CELL CULTURES
8 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE
8.1 OVERVIEW
8.2 SERVICES
8.3 PRODUCT
8.3.1 CHO-K1
8.3.1.1 CHO-K1 ATCC
8.3.1.2 CHO-K1 ECACC
8.3.1.3 Others
8.3.2 CHO-DG44
8.3.3 CHO-S
8.3.4 CHO-DXB11
8.3.5 OTHERS
9 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM
9.1 OVERVIEW
9.2 METABOLIC SELECTION SYSTEM
9.3 ANTIBIOTIC SELECTION SYSTEM
9.4 OTHERS
10 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION
10.1 OVERVIEW
10.2 BIOLOGICS
10.2.1 MONOCLONAL ANTIBODIES
10.2.2 FC-FUSION PROTIEN
10.2.3 ENZYMES
10.2.4 HORMONES
10.2.5 CYTOKINES
10.2.6 CLOTTING FACTORS
10.2.7 OTHERS
10.3 MEDICAL RESEARCH
11 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER
11.1 OVERVIEW
11.2 BIOPHARMACEUTICAL COMPANIES
11.2.1 MEDIUM
11.2.2 SMALL
11.3 CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS
11.3.1 MEDIUM
11.3.2 SMALL
11.4 BIOTECHNOLOGY COMPANIES
11.4.1 MEDIUM
11.4.2 SMALL
11.5 ACADEMIC INSTITUTES AND RESEARCH ORGANIZATIONS
11.6 CLINICAL RESEARCH ORGANIZATIONS
11.6.1 MEDIUM
11.6.2 SMALL
11.7 OTHERS
12 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDERS
12.3 RETAIL SALES
12.4 OTHERS
13 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 SOUTH KOREA
13.1.4 INDIA
13.1.5 AUSTRALIA
13.1.6 TAIWAN
13.1.7 SINGAPORE
13.1.8 HONG KONG
13.1.9 THAILAND
13.1.10 MALAYSIA
13.1.11 INDONESIA
13.1.12 PHILIPPINES
13.1.13 NEW ZEALAND
13.1.14 REST OF ASIA-PACIFIC
14 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 SARTORIUS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 LONZA
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENT
16.3 THERMO FISHER SCIENTIFIC INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 CYTIVA
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 MERCK KGAA
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 ABEOMICS
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENT
16.7 ACCEGEN
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 APPLIED BIOLOGICAL MATERIALS INC. (ABM)
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ATCC
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 BPS BIOSCIENCE, INC.
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 CYTION
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 CURIA GLOBAL, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 SERVICE PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 GENTARGET INC.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 GTP BIOWAYS
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENT
16.15 PROMEGA CORPORATION
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
16.16 RAYBIOTECH LIFE, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 REVVITY DISCOVERY LIMITED.
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 ASIA-PACIFIC SERVICES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 ASIA-PACIFIC PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 4 ASIA-PACIFIC PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 ASIA-PACIFIC CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 7 ASIA-PACIFIC METABOLIC SELECTION SYSTEM IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 8 ASIA-PACIFIC ANTIBIOTIC SELECTION SYSTEM IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 ASIA-PACIFIC OTHERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 10 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 11 ASIA-PACIFIC BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 ASIA-PACIFIC BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 13 ASIA-PACIFIC MEDICAL RESEARCH IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 14 ASIA-PACIFIC CHINESE HAMSTER OVARY CELLS CHO MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 15 ASIA-PACIFIC BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 16 ASIA-PACIFIC BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 ASIA-PACIFIC CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 ASIA-PACIFIC CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 19 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 21 ASIA-PACIFIC ACADEMIC INSTITUTES AND RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 ASIA-PACIFIC CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 ASIA-PACIFIC CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 24 ASIA-PACIFIC OTHERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 ASIA-PACIFIC DIRECT TENDERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 ASIA-PACIFIC RETAIL SALES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 ASIA-PACIFIC OTHERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 29 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 30 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 31 ASIA-PACIFIC PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 32 ASIA-PACIFIC CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 34 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 35 ASIA-PACIFIC BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 37 ASIA-PACIFIC BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 38 ASIA-PACIFIC CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 39 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 40 ASIA-PACIFIC CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 42 CHINA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 CHINA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 CHINA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 CHINA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 46 CHINA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 47 CHINA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 48 CHINA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 49 CHINA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 CHINA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 CHINA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 52 CHINA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 CHINA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 54 JAPAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 JAPAN PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 JAPAN CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 JAPAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 58 JAPAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 59 JAPAN BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 JAPAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 61 JAPAN BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 JAPAN CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 JAPAN BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 JAPAN CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 JAPAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 66 SOUTH KOREA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 SOUTH KOREA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 SOUTH KOREA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 SOUTH KOREA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 70 SOUTH KOREA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 71 SOUTH KOREA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 SOUTH KOREA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 SOUTH KOREA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 SOUTH KOREA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 SOUTH KOREA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 SOUTH KOREA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 SOUTH KOREA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 78 INDIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 INDIA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 INDIA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 INDIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 82 INDIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 83 INDIA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 INDIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 85 INDIA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 86 INDIA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 INDIA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 INDIA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 INDIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 90 AUSTRALIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 AUSTRALIA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 92 AUSTRALIA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 AUSTRALIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 94 AUSTRALIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 95 AUSTRALIA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 96 AUSTRALIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 97 AUSTRALIA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 AUSTRALIA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 AUSTRALIA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 AUSTRALIA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 AUSTRALIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 102 TAIWAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 TAIWAN PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 104 TAIWAN CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 TAIWAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 106 TAIWAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 107 TAIWAN BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 TAIWAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 109 TAIWAN BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 TAIWAN CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 111 TAIWAN BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 TAIWAN CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 TAIWAN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 114 SINGAPORE CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 SINGAPORE PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 SINGAPORE CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 SINGAPORE CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 118 SINGAPORE CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 119 SINGAPORE BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 SINGAPORE CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 121 SINGAPORE BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 SINGAPORE CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 123 SINGAPORE BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 124 SINGAPORE CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 SINGAPORE CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 126 HONG KONG CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 HONG KONG PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 128 HONG KONG CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 HONG KONG CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 130 HONG KONG CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 131 HONG KONG BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 HONG KONG CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 133 HONG KONG BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 134 HONG KONG CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 HONG KONG BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 HONG KONG CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 HONG KONG CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 138 THAILAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 THAILAND PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 THAILAND CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 THAILAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 142 THAILAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 143 THAILAND BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 THAILAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 145 THAILAND BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 THAILAND CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 147 THAILAND BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 THAILAND CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 149 THAILAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 150 MALAYSIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 MALAYSIA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 MALAYSIA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 153 MALAYSIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 154 MALAYSIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 155 MALAYSIA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 MALAYSIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 157 MALAYSIA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 MALAYSIA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 MALAYSIA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 MALAYSIA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 161 MALAYSIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 162 INDONESIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 INDONESIA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 INDONESIA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 INDONESIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 166 INDONESIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 167 INDONESIA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 168 INDONESIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 169 INDONESIA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 INDONESIA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 INDONESIA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 172 INDONESIA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 INDONESIA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 174 PHILIPPINES CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 PHILIPPINES PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 PHILIPPINES CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 PHILIPPINES CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 178 PHILIPPINES CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 179 PHILIPPINES BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 180 PHILIPPINES CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 181 PHILIPPINES BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 PHILIPPINES CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 183 PHILIPPINES BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 PHILIPPINES CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 185 PHILIPPINES CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 186 NEW ZEALAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 187 NEW ZEALAND PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 188 NEW ZEALAND CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 189 NEW ZEALAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 190 NEW ZEALAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 191 NEW ZEALAND BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 NEW ZEALAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 193 NEW ZEALAND BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 NEW ZEALAND CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 NEW ZEALAND BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 NEW ZEALAND CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 NEW ZEALAND CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 198 REST OF ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Liste des figures
FIGURE 1 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: ASIA-PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EXECUTIVE SUMMARY: ASIA-PACIFIC CHINESE HAMSTER OVARY CELLS CHO MARKET
FIGURE 11 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: SEGMENTATION
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 TWO SEGMENTS COMPRISE THE ASIA-PACIFIC CHINESE HAMSTER OVARY CELLS CHO MARKET, BY TYPE
FIGURE 14 RISING DEMAND FOR BIOLOGICS AND THERAPEUTIC PROTEINS EXPECTED TO DRIVE THE ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 15 TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 16 NUMBER OF PATENTS PER COUNTRY OR REGION
FIGURE 17 NUMBER OF PATENTS PER APPLICANTS
FIGURE 18 NUMBER OF PATENTS PER YEAR.
FIGURE 19 DROC ANALYSIS
FIGURE 20 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, 2024
FIGURE 21 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, 2025 TO 2032 (USD THOUSAND)
FIGURE 22 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, CAGR (2025- 2032)
FIGURE 23 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, 2024
FIGURE 25 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, 2025 TO 2032 (USD THOUSAND)
FIGURE 26 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, CAGR (2025- 2032)
FIGURE 27 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, 2024
FIGURE 29 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 30 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, CAGR (2025- 2032)
FIGURE 31 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, 2024
FIGURE 33 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 34 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, CAGR (2025- 2032)
FIGURE 35 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 37 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, 2025 TO 2032 (USD THOUSAND)
FIGURE 38 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025- 2032)
FIGURE 39 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: SNAPSHOT (2022)
FIGURE 41 ASIA-PACIFIC CHINESE HAMSTER OVARY (CHO) CELLS MARKET: COMPANY SHARE 2024 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.