Las pruebas en el punto de atención (POCT) revolucionan la prestación de atención médica al permitir diagnósticos rápidos en la ubicación del paciente. Sus aplicaciones abarcan diversos entornos médicos, incluidas clínicas, hospitales y áreas remotas. Los dispositivos POCT ofrecen interfaces fáciles de usar, lo que produce resultados rápidos y precisos para afecciones como diabetes, marcadores cardíacos y enfermedad infecciosa. Estas características mejoran la atención al paciente al facilitar decisiones de tratamiento inmediatas, reducir los tiempos de respuesta y ampliar el acceso a la atención médica a regiones desatendidas, optimizando en última instancia los resultados médicos.
Acceder al informe completo @https://www.databridgemarketresearch.com/reports/europe-poct-market
Data Bridge Market Research analiza que el Mercado europeo de pruebas en el punto de atención (POCT) está valorado en 6.664,28 millones de dólares en 2021 y se espera que alcance los 13.085,33 millones de dólares en 2029, registrando una tasa compuesta anual del 8,8% durante el período previsto de 2022 a 2029. Las pruebas en el punto de atención (POCT) proporcionan resultados de diagnóstico rápidos directamente en la ubicación del paciente , lo que permite a los profesionales sanitarios tomar decisiones de tratamiento inmediatas. Este rápido acceso a información crítica mejora la atención al paciente al minimizar los retrasos en el diagnóstico y la intervención, lo que conduce a una gestión médica más oportuna y eficaz.
Hallazgos clave del estudio
Se espera que el diagnóstico y la intervención tempranos impulsen la tasa de crecimiento del mercado.
Las pruebas en el lugar de atención (POCT) desempeñan un papel fundamental en la identificación temprana de enfermedades, lo que permite un inicio rápido de los tratamientos y, en última instancia, conduce a mejores resultados para los pacientes. Al proporcionar información de diagnóstico rápida e inmediata, los profesionales de la salud pueden intervenir rápidamente con terapias adecuadas, previniendo la progresión de la enfermedad y sus complicaciones. Este enfoque proactivo mejora significativamente la eficacia de las intervenciones médicas, mejora el pronóstico de los pacientes y contribuye a la eficiencia general del sistema sanitario.
Alcance del informe y segmentación del mercado
|
Métrica de informe
|
Detalles
|
|
Período de pronóstico
|
2022 a 2029
|
|
Año base
|
2021
|
|
Años históricos
|
2020 (Personalizable para 2014-2019)
|
|
Unidades Cuantitativas
|
Ingresos en millones de dólares, volúmenes en unidades, precios en dólares
|
|
Segmentos cubiertos
|
Productos (Instrumentos, Consumibles y Reactivos y Otros), Aplicación (Glucosa en Sangre, Monitoreo Cardíaco, Coagulación, Análisis de Sangre Total, Monitoreo de Signos Vitales, Pruebas de Enfermedades Infecciosas, Análisis de Orina, Pruebas de colesterol y otros), usuario final (laboratorios, hospitales, clínicas, centros ambulatorios, atención domiciliaria, centros de vida asistida, farmacias/droguerías y otros), modo de prueba (pruebas con receta y pruebas sin receta), canal de distribución (ventas directas, terceros). Distribuidor de Fiestas y Venta Online)
|
|
Países cubiertos
|
Alemania, Francia, Reino Unido, Países Bajos, Suiza, Bélgica, Rusia, Italia, España, Turquía, Resto de Europa en Europa
|
|
Actores del mercado cubiertos
|
Siemens Healthcare GmbH (Alemania), F. Hoffmann-La Roche Ltd. (Suiza), Quidel Corporation (EE.UU.), Trividia Health, Inc. (EE.UU.), Nova Biomedical (EE.UU.), PTS Diagnostics (EE.UU.), Trinity Biotech (Irlanda) ), LumiraDx (Reino Unido), Sekisui Diagnostics (EE.UU.), Chembio Diagnostics, Inc. (EE.UU.), BD (EE.UU.), Thermo Fisher Scientific Inc. (EE.UU.), Abbott (EE.UU.), Danaher (EE.UU.), binx health, inc. (EE.UU.), QuantuMDx Group Ltd. (Reino Unido), Werfen (España), Abaxis (EE.UU.), EKF Diagnostics (Reino Unido), Sysmex Europe SE (Alemania)
|
|
Puntos de datos cubiertos en el informe
|
Además de la información sobre escenarios de mercado como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis profundos de expertos, epidemiología de pacientes, análisis de tuberías, análisis de precios, y marco regulatorio
|
Análisis de segmentos:
El mercado europeo de pruebas en el punto de atención (POCT) está segmentado según el producto, la aplicación, el usuario final, el modo de prueba y el canal de distribución.
- Según el producto, el mercado se segmenta en instrumentos, consumibles y reactivos, y otros.
- Según la aplicación, el mercado se segmenta en glucosa en sangre, monitorización cardíaca, coagulación, análisis de sangre completa, monitorización de signos vitales, pruebas de enfermedades infecciosas, análisis de orina, pruebas de colesterol y otros.
- Según el usuario final, el mercado se segmenta en laboratorios, hospitales, clínicas, centros ambulatorios, atención domiciliaria, instalaciones de vida asistida, farmacias/droguerías y otros.
- Según el modo de realización de las pruebas, el mercado se segmenta en pruebas con receta y pruebas sin receta.
- Según el canal de distribución, el mercado se segmenta en ventas directas, distribuidores externos y ventas en línea.
Principales actores
Data Bridge Market Research reconoce a las siguientes empresas como los principales actores del mercado europeo de pruebas en el punto de atención (POCT) Siemens Healthcare GmbH (Alemania), F. Hoffmann-La Roche Ltd. (Suiza), Quidel Corporation (EE. UU.), Trividia Health, Inc. (EE. UU.), Nova Biomedical (EE. UU.), PTS Diagnostics (EE. UU.), Trinity Biotech (Irlanda), LumiraDx (Reino Unido),
Desarrollos del mercado
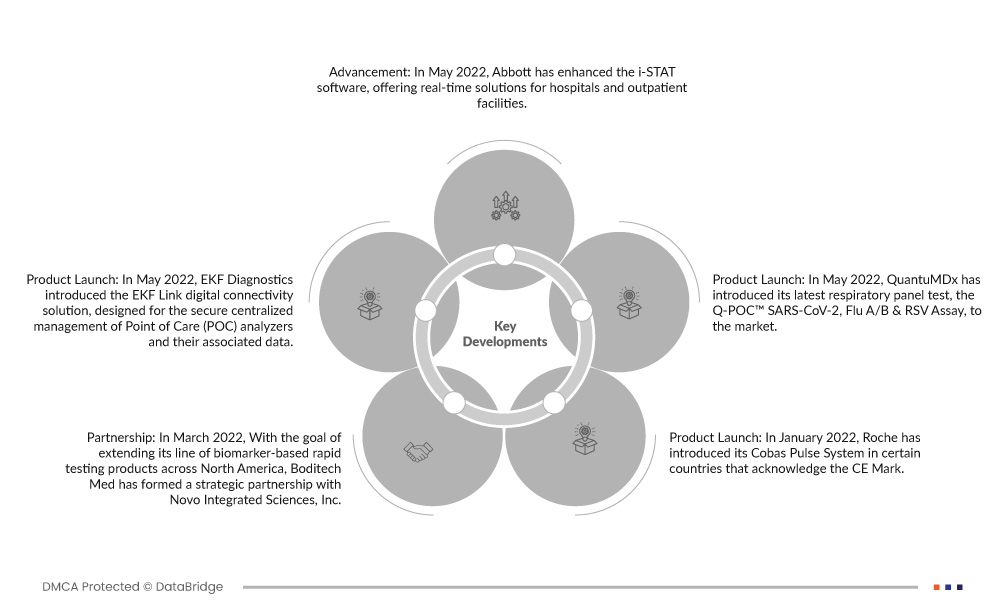
- En mayo de 2022, Abbott mejoró el software i-STAT y ofrece soluciones en tiempo real para hospitales e instalaciones para pacientes ambulatorios. Este software actualizado cuenta con una alta tasa de eficacia, rentabilidad y respalda la toma de decisiones de atención de calidad al paciente, lo que garantiza soluciones de atención médica eficientes y precisas.
- En mayo de 2022, EKF Diagnostics presentó la solución de conectividad digital EKF Link, diseñada para la gestión centralizada segura de analizadores Point of Care (POC) y sus datos asociados. Esta plataforma agiliza el proceso de gestión, garantiza la seguridad de los datos y mejora la eficiencia de las operaciones de prueba POC.
- En mayo de 2022, QuantuMDx introdujo en el mercado su última prueba de panel respiratorio, el ensayo Q-POC™ SARS-CoV-2, Flu A/B & RSV.
- En marzo de 2022, con el objetivo de ampliar su línea de productos de prueba rápida basados en biomarcadores en América del Norte, Boditech Med formó una asociación estratégica con Novo Integrated Sciences, Inc.
- En enero de 2022, Roche introdujo su sistema Cobas Pulse en determinados países que reconocen la marca CE. Esta oferta avanzada de Roche Diagnostics representa su última solución conectada para el punto de atención diseñada para el control profesional de la glucosa en sangre.
Análisis Regional
Geográficamente, los países cubiertos en el informe del mercado europeo de pruebas en el punto de atención (POCT) son Alemania, Francia, Reino Unido, Países Bajos, Suiza, Bélgica, Rusia, Italia, España, Turquía y el resto de Europa en Europa.
Según el análisis de investigación de mercado de Data Bridge:
Alemania es la región dominante en Europa Mercado de pruebas en el punto de atención (POCT) durante el período de pronóstico 2022 - 2029
En 2022, Alemania dominó el mercado de pruebas en el punto de atención (POCT) debido a la creciente conciencia que abarca los beneficios de las pruebas in situ, ofreciendo resultados rápidos que mejoran la atención al paciente, agilizan las opciones de tratamiento y mejoran el manejo de enfermedades. Esta mayor familiaridad con las capacidades de POCT impulsa su adopción como un elemento fundamental de las prácticas sanitarias contemporáneas en toda Europa.
Para obtener información más detallada sobre el mercado de pruebas en el punto de atención (POCT) informe, haga clic aquí – https://www.databridgemarketresearch.com/reports/europe-poct-market