Globaler Markt für Sepsisdiagnostik , nach Techniken (Immunoassay, Molekulardiagnostik , Mikrobiologie und Durchflusszytometrie), Testtyp (Labortests und Point-of- Care -Tests), Branchentrends und Prognose bis 2029.
Globale Marktanalyse und Einblicke: Globaler Markt für Sepsisdiagnostik
Sepsis ist ein potenziell lebensbedrohlicher Zustand, der auftritt, wenn die Reaktion des Körpers auf eine Infektion sein eigenes Gewebe schädigt. Wenn sich die Infektionsbekämpfungsprozesse gegen den Körper wenden, führen sie dazu, dass die Organe schlecht und abnormal funktionieren. Sepsis kann zu einem septischen Schock führen. Dabei handelt es sich um einen dramatischen Blutdruckabfall, der zu schweren Organproblemen und zum Tod führen kann. Um Sepsis zu diagnostizieren, muss die Infektion durch Anzeichen wie Veränderungen des Geisteszustands, des systolischen Blutdrucks und der Atemfrequenz bestätigt werden. Am häufigsten tritt Sepsis bei Patienten auf, die im Krankenhaus sind oder vor Kurzem im Krankenhaus waren. Patienten auf einer Intensivstation entwickeln häufiger Infektionen, die dann zu Sepsis führen können. Zur Diagnose einer Sepsis müssen neben Bluttests auch andere Labortests durchgeführt werden. Blutproben werden verwendet, um auf Anzeichen einer Infektion, Gerinnungsprobleme, abnormale Leber- oder Nierenfunktion, beeinträchtigte Sauerstoffversorgung und Elektrolytstörungen zu testen. Patienten mit Sepsis müssen engmaschig überwacht und auf einer Intensivstation behandelt werden. Möglicherweise sind lebensrettende Maßnahmen erforderlich, um die Atmung und Herzfunktion zu stabilisieren.
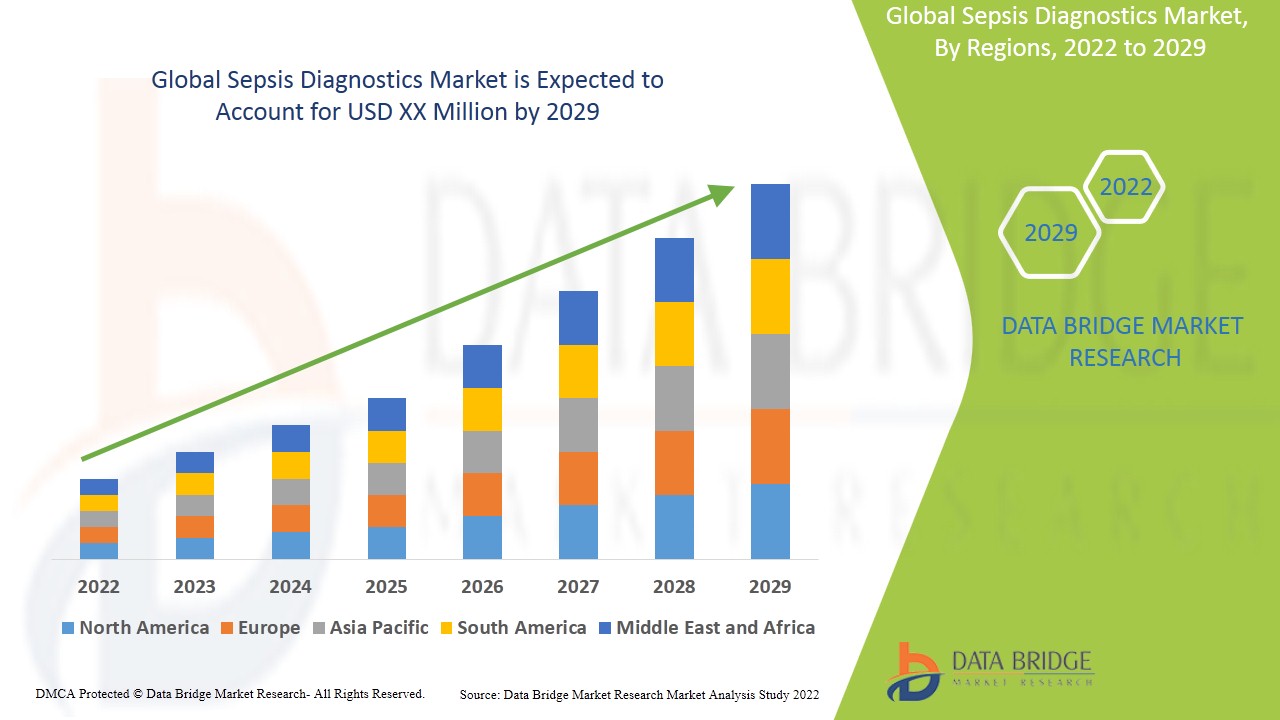
Data Bridge Market Research analysiert, dass der Markt im Prognosezeitraum von 2022 bis 2029 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 8,0 % wächst.
|
Berichtsmetrik |
Details |
|
Prognosezeitraum |
2022 bis 2029 |
|
Basisjahr |
2021 |
|
Historische Jahre |
2019–2020 (anpassbar auf 2019–2014) |
|
Quantitative Einheiten |
Umsatz in Mio. USD, Mengen in Einheiten, Preise in USD |
|
Abgedeckte Segmente |
Nach Techniken (Immunoassay, Molekulardiagnostik, Mikrobiologie und Durchflusszytometrie), Testtyp (Labortests und Point-of-Care-Tests) |
|
Abgedeckte Länder |
USA und Rest von Nordamerika in Nordamerika, Deutschland, Frankreich, Großbritannien und Rest von Europa in Europa, China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, Philippinen, Rest von Asien-Pazifik (APAC) im Asien-Pazifik-Raum (APAC), Brasilien und Rest von Südamerika als Teil von Südamerika. Südafrika, Saudi-Arabien und Rest von Naher Osten und Afrika (MEA) als Teil von Naher Osten und Afrika (MEA). |
|
Abgedeckte Marktteilnehmer |
Die wichtigsten Unternehmen, die auf dem Markt tätig sind, sind Trinity Biotech (Irland), Meridian Bioscience (USA), Omega Diagnostics Group PLC. (Großbritannien), Xcyton Diagnostics Limited (Indien), Diasorin SpA (Italien), Seegene Inc. (Südkorea), EKF Diagnostics Holdings plc (Großbritannien), Axis-Shield Diagnostics Ltd. (Großbritannien), Immunexpress Inc. (USA), Luminex Corporation (USA), bioMérieux SA (Frankreich), BD (USA), Thermo Fisher Scientific Inc. (USA), Abbott (USA), Roche Diagnostics (USA), Cepheid (USA), Beckman Coulter, Inc. (USA), T2 Biosystems, Inc. (USA), Bruker (USA) und Ortho Clinical Diagnostics (USA). |
Marktdynamik für Sepsisdiagnostik
Treiber
- Steigende Zahl von Krankenhausinfektionen
Mit der rasanten Zunahme von Krankenhausinfektionen weltweit steigt in den kommenden Jahren auch die Nachfrage nach geeigneten Sepsis-Diagnostikprodukten. Daher wird erwartet, dass die steigende Zahl von Krankenhausinfektionen das Wachstum des Sepsis-Diagnostikmarktes vorantreiben wird.
- Steigende Gesundheitsausgaben
Ein weiterer wichtiger Faktor, der die Wachstumsrate des Marktes für Sepsisdiagnostik beeinflusst, sind die steigenden Gesundheitsausgaben, die zur Verbesserung der Infrastruktur beitragen.
- Steigende Prävalenz der Sepsis
Die zunehmende Verbreitung von Sepsis in verschiedenen Regionen weltweit und die damit verbundenen verschiedenen Infektionen dürften als Wachstumstreiber für den Markt für Sepsisdiagnostik wirken.
Darüber hinaus sind Fortschritte in der Medizintechnik, zunehmende Initiativen öffentlicher und privater Organisationen zur Sensibilisierung und wachsende staatliche Finanzierung Faktoren, die den Markt für Sepsisdiagnostik erweitern werden.
Gelegenheiten
- Entwicklung neuer Biomarker für die Sepsisdiagnose
Sepsis ist eine der Hauptursachen für Mortalität und Morbidität, auch wenn heute Breitbandantibiotika und moderne medizinische Versorgung verfügbar sind. Biomarker sind ein Werkzeug, um eine frühzeitige Diagnose zu ermöglichen, Patientengruppen mit hohem Komplikationsrisiko zu identifizieren und den Krankheitsverlauf zu überwachen. Dies sind wichtige Beurteilungen für eine geeignete Therapie und eine Verbesserung der Patientenergebnisse.
Darüber hinaus wird die Einführung wirksamer Therapien und fortlaufender klinischer Studien dem Markt für Sepsisdiagnostik im Prognosezeitraum 2022–2029 gewinnbringende Chancen bieten. Darüber hinaus werden der hohe ungedeckte Bedarf an aktuellen Behandlungen und Entwicklungen in der Gesundheitstechnologie das Wachstum des Marktes für Sepsisdiagnostik in Zukunft beschleunigen.
Einschränkungen/Herausforderungen
Die hohen Diagnosekosten und der Mangel an geeigneten Tests für Sepsis werden jedoch das Wachstum des Sepsisdiagnostikmarktes hemmen. Darüber hinaus wird das mangelnde Bewusstsein für Sepsis den Markt im oben genannten Prognosezeitraum vor weitere Herausforderungen stellen.
Dieser Marktbericht zur Sepsisdiagnostik enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-Export-Analysen, Produktionsanalysen, Optimierung der Wertschöpfungskette, Marktanteilen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neu entstehende Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Markt für Sepsisdiagnostik zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analystenbericht zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Auswirkungen von COVID-19 auf den Markt für Sepsisdiagnostik
COVID-19 hat sich negativ auf den Markt ausgewirkt. Lockdowns und Isolation während einer Pandemie erschweren das Krankheitsmanagement und die Einhaltung der Medikamenteneinnahme. Der fehlende Zugang zu Gesundheitseinrichtungen für Routinebehandlungen und Medikamentenverabreichung wird den Markt weiter beeinträchtigen. Soziale Isolation erhöht Stress, Verzweiflung und soziale Unterstützung, was alles zu einer Verringerung der Einhaltung der Sepsis-Medikamenten während der Pandemie führen kann.
Jüngste Entwicklung
- Im Mai 2020 gab Beckman Coulter die Verfügbarkeit seines Hämatologieanalysators DxH 690T in den USA bekannt, der als Frühindikator für Sepsis eingesetzt wird. Diese Ankündigung hat dem Unternehmen geholfen, seine globale Präsenz zu steigern und auch den Umsatz zu steigern.
 Globaler Marktumfang für Sepsisdiagnostik
Globaler Marktumfang für Sepsisdiagnostik
Der globale Markt für Sepsisdiagnostik ist in Techniken und Testtypen unterteilt. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, um strategische Entscheidungen zur Identifizierung der wichtigsten Marktanwendungen zu treffen.
Techniken
- Immunassay
- Molekulare Diagnostik
- Mikrobiologie
- Durchflusszytometrie
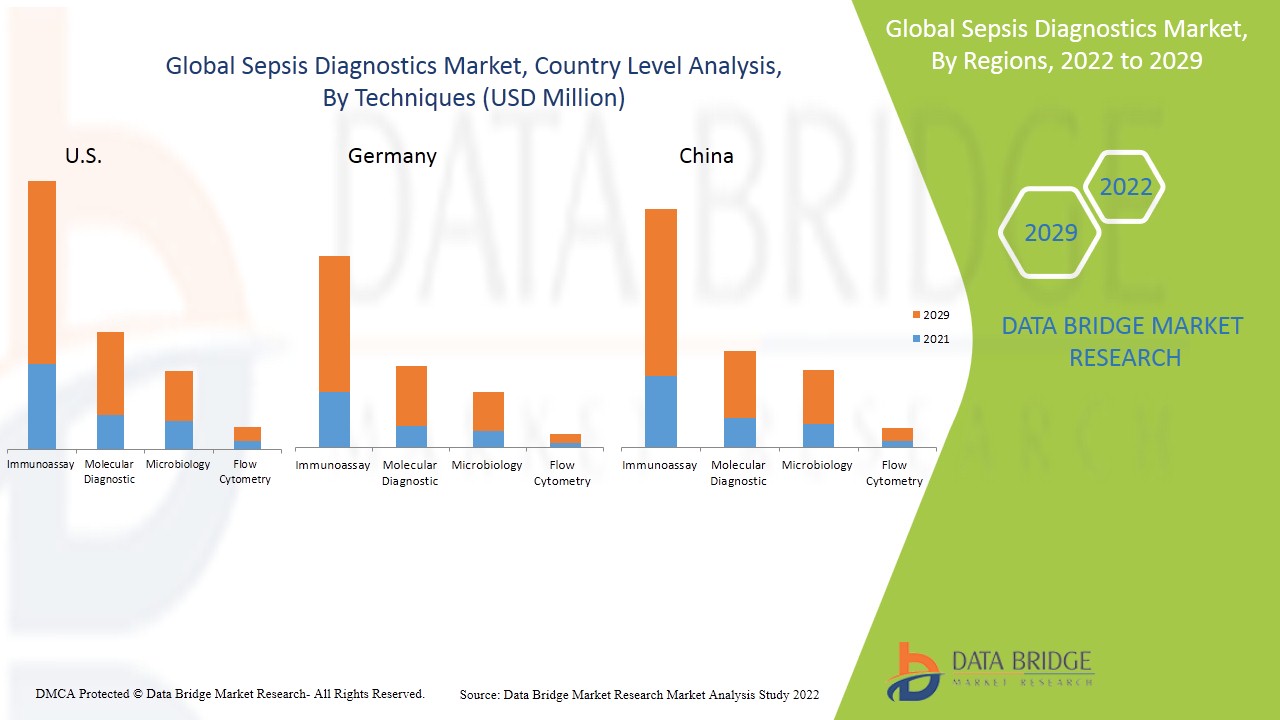
Auf der Grundlage der Techniken ist der globale Markt für Sepsisdiagnostik in Mikrobiologie, Molekulardiagnostik, Immunassay und Durchflusszytometrie segmentiert.
Testtyp
- Labortests
- Point-of-Care-Diagnostik
Auf der Grundlage des Testtyps ist der globale Markt für Sepsisdiagnostik in Labortests und Point-of-Care-Tests segmentiert.
Regionale Analyse/Einblicke zum Sepsisdiagnostikmarkt
Der Markt für Sepsisdiagnostik wird analysiert und es werden Einblicke in die Marktgröße und Trends nach Land, Techniken und Testtyp, wie oben angegeben, bereitgestellt.
Die im Marktbericht zur Sepsisdiagnostik abgedeckten Länder sind die USA, der Rest von Nordamerika, Deutschland, Großbritannien, Frankreich, der Rest von Europa, China, Indien, Japan, Südkorea, Australien, Singapur, Thailand, Malaysia, Indonesien, die Philippinen, der Rest des asiatisch-pazifischen Raums, Brasilien, der Rest von Südamerika, Saudi-Arabien, Südafrika sowie der Rest des Nahen Ostens und Afrikas.
Nordamerika dominiert den Markt für Sepsisdiagnostik in Bezug auf Marktanteil und Marktumsatz und wird seine Dominanz im Prognosezeitraum weiter ausbauen. Dies ist auf die Präsenz wichtiger Schlüsselakteure und eine gut entwickelte Gesundheitsinfrastruktur in dieser Region zurückzuführen, zusammen mit der steigenden Zahl der erwachsenen Bevölkerung, bei der in der Region jedes Jahr Sepsis diagnostiziert wird. Der asiatisch-pazifische Raum hingegen wird im Prognosezeitraum voraussichtlich die höchste Wachstumsrate aufweisen, da die Regierungen der Entwicklungsländer ihre Gesundheitsausgaben erhöhen und die staatliche Unterstützung zunimmt.
Der Länderabschnitt des Berichts enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Marktvorschriften, die sich auf die aktuellen und zukünftigen Markttrends auswirken. Datenpunkte wie Neu- und Ersatzverkäufe, demografische Daten des Landes, Krankheitsepidemiologie und Import- und Exportzölle sind einige der wichtigsten Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Darüber hinaus werden bei der Prognoseanalyse der Länderdaten die Präsenz und Verfügbarkeit globaler Marken und ihre Herausforderungen aufgrund der hohen Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen der Vertriebskanäle berücksichtigt.
Wettbewerbsumfeld und Sepsisdiagnostik Marktanteilsanalyse
Die Wettbewerbslandschaft auf dem Sepsisdiagnostikmarkt liefert Einzelheiten zu den Wettbewerbern. Die enthaltenen Details sind Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen auf den Sepsisdiagnostikmarkt .
Zu den wichtigsten Akteuren auf dem Markt für Sepsisdiagnostik zählen unter anderem Trinity Biotech (Irland), Meridian Bioscience, Omega Diagnostics Group PLC., Xcyton Diagnostics Limited, Diasorin SpA, Seegene Inc., EKF Diagnostics Holdings plc., Axis-Shield Diagnostics Ltd., Immunexpress Inc., Luminex Corporation, bioMérieux SA, BD, Thermo Fisher Scientific Inc. Abbott, Roche Diagnostics, Cepheid, Beckman Coulter, Inc., T2 Biosystems, Inc. und Bruker Ortho Clinical Diagnostics.
Forschungsmethodik : Globaler Markt für Sepsisdiagnostik
Die Datenerfassung und die Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Die wichtigste Forschungsmethode, die das DBMR-Forschungsteam verwendet, ist die Datentriangulation, die Data Mining, Analyse der Auswirkungen von Datenvariablen auf den Markt und primäre (Branchenexperten-)Validierung umfasst. Abgesehen davon umfassen die Datenmodelle ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, einen Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Firmenmarktanteilsanalyse, Messstandards, globale vs. regionale und Lieferantenanteilsanalyse. Bitte fordern Sie bei weiteren Fragen einen Analystenanruf an.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL SEPSIS DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TECHNIQUES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TECHNIQUES COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN INCIDENCE OF HOSPITAL-ACQUIRED INFECTIONS
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 GROW IN PREVALENCE OF SEPSIS
5.1.4 RISE IN TECHNOLOGICAL ADVANCEMENTS OF SEPSIS DIAGNOSTIC DEVICES
5.2 RESTRAINTS
5.2.1 HIGH COST OF DIAGNOSIS
5.2.2 LACK OF APPROPRIATE TESTING FOR SEPSIS
5.3 OPPORTUNITIES
5.3.1 DEVELOPMENT OF RAPID DIAGNOSTIC/POINT OF CARE (POC) TECHNIQUES FOR EARLY SEPSIS DIAGNOSIS
5.3.2 EVOLUTION OF NOVEL BIOMARKERS FOR SEPSIS DIAGNOSIS
5.4 CHALLENGES
5.4.1 LACK OF AWARENESS ABOUT SEPSIS
5.4.2 SHORTAGE OF SKILLED HEALTHCARE PROFESSIONALS
6 GLOBAL SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES
6.1 OVERVIEW
6.2 IMMUNOASSAY
6.2.1 PROCALCITONIN (PCT)
6.2.2 INTERLEUKIN-6 (IL-6)
6.2.3 C-REACTIVE PROTEIN (CRP)
6.2.4 PENTRAXIN-3 (PTX3)
6.2.5 CALPROTECTIN
6.2.6 OTHERS
6.3 MOLECULAR DIAGNOSTIC
6.4 MICROBIOLOGY
6.5 FLOW CYTOMETRY
7 GLOBAL SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 LABORATORY TESTING
7.3 POINT OF CARE TESTING
8 GLOBAL SEPSIS DIAGNOSTICS MARKET, BY REGION
8.1 OVERVIEW
8.2 NORTH AMERICA
8.2.1 OVERVIEW
8.2.2 U.S.
8.2.3 REST OF NORTH AMERICA
8.3 EUROPE
8.3.1 OVERVIEW
8.3.2 GERMANY
8.3.3 U.K.
8.3.4 FRANCE
8.3.5 REST OF EUROPE
8.4 ASIA-PACIFIC
8.4.1 OVERVIEW
8.4.2 CHINA
8.4.3 INDIA
8.4.4 JAPAN
8.4.5 SOUTH KOREA
8.4.6 AUSTRALIA
8.4.7 SINGAPORE
8.4.8 THAILAND
8.4.9 MALAYSIA
8.4.10 INDONESIA
8.4.11 PHILIPPINES
8.4.12 REST OF ASIA-PACIFIC
8.5 SOUTH AMERICA
8.5.1 OVERVIEW
8.5.2 BRAZIL
8.5.3 REST OF SOUTH AMERICA
8.6 MIDDLE EAST AND AFRICA
8.6.1 OVERVIEW
8.6.2 SOUTH AFRICA
8.6.3 SAUDI ARABIA
8.6.4 REST OF MIDDLE EAST AND AFRICA
9 GLOBAL SEPSIS DIAGNOSTICS MARKET: COMPANY LANDSCAPE
9.1 COMPANY SHARE ANALYSIS: GLOBAL
9.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
9.3 COMPANY SHARE ANALYSIS: EUROPE
9.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
10 COMPANY PROFILE
10.1 ROCHE DIAGNOSTICS
10.1.1 COMPANY SNAPSHOT
10.1.2 REVENUE ANALYSIS
10.1.3 COMPANY SHARE ANALYSIS
10.1.4 PRODUCT PORTFOLIO
10.1.5 RECENT DEVELOPMENT
10.2 ABBOTT.
10.2.1 COMPANY SNAPSHOT
10.2.2 REVENUE ANALYSIS
10.2.3 COMPANY SHARE ANALYSIS
10.2.4 PRODUCT PORTFOLIO
10.2.5 RECENT DEVELOPMENT
10.2.5.1 PRODUCT LAUNCH
10.3 BIOMERIEUX SA
10.3.1 COMPANY SNAPSHOT
10.3.2 REVENUE ANALYSIS
10.3.3 COMPANY SHARE ANALYSIS
10.3.4 PRODUCT PORTFOLIO
10.3.5 RECENT DEVELOPMENT
10.3.5.1 PRODUCT LAUNCH
10.4 THERMO FISHER SCINETIFIC INC.
10.4.1 COMPANY SNAPSHOT
10.4.2 REVENUE ANALYSIS
10.4.3 COMPANY SHARE ANALYSIS
10.4.4 PRODUCT PORTFOLIO
10.4.5 RECENT DEVELOPMENT
10.4.5.1 ACQUISITION
10.5 BD
10.5.1 COMPANY SNAPSHOT
10.5.2 REVENUS ANALYSIS
10.5.3 COMPANY SHARE ANALYSIS
10.5.4 PRODUCT PORTFOLIO
10.5.5 RECENT DEVELOPMENT
10.6 SEEGENE INC.
10.6.1 COMPANY SNAPSHOT
10.6.2 REVENUE ANALYSIS
10.6.3 PRODUCT PORTFOLIO
10.6.4 RECENT DEVELOPMENT
10.7 AXIS-SHIELD DIAGNOSTICS LTD.
10.7.1 COMPANY SNAPSHOT
10.7.2 PRODUCT PORTFOLIO
10.7.3 RECENT DEVELOPMENTS
10.8 BECKMAN COULTER, INC.
10.8.1 COMPANY SNAPSHOT
10.8.2 PRODUCT PORTFOLIO
10.8.3 RECENT DEVELOPMENTS
10.9 BRUKER
10.9.1 COMPANY SNAPSHOT
10.9.2 REVENUS ANALYSIS
10.9.3 PRODUCT PORTFOLIO
10.9.4 RECENT DEVELOPMENTS
10.1 CEPHEID
10.10.1 COMPANY SNAPSHOT
10.10.2 REVENUE ANALYSIS
10.10.3 PRODUCT PORTFOLIO
10.10.4 RECENT DEVELOPMENT
10.10.4.1 PROGRAM LAUNCH
10.11 DIASORIN S.P.A.
10.11.1 COMPANY SNAPSHOT
10.11.2 REVENUE ANALYSIS
10.11.3 PRODUCT PORTFOLIO
10.11.4 RECENT DEVELOPMENT
10.11.4.1 ACQUISITION
10.12 EKF DIAGNOSTICS HOLDINGS PLC
10.12.1 COMPANY SNAPSHOT
10.12.2 REVENUE ANALYSIS
10.12.3 PRODUCT PORTFOLIO
10.12.4 RECENT DEVELOPMENT
10.12.4.1 ACQUISITION
10.13 IMMUNEXPRESS INC.
10.13.1 COMPANY SNAPSHOT
10.13.2 PRODUCT PORTFOLIO
10.13.3 RECENT DEVELOPMENT
10.13.3.1 PRODUCT LAUNCH
10.14 LUMINEX CORPORATION.
10.14.1 COMPANY SNAPSHOT
10.14.2 PRODUCT PORTFOLIO
10.14.3 RECENT DEVELOPMENT
10.14.3.1 ACQUISITION
10.15 MERIDIAN BIOSCIENCE
10.15.1 COMPANY SNAPSHOT
10.15.2 REVENUS ANALYSIS
10.15.3 PRODUCT PORTFOLIO
10.15.4 RECENT DEVELOPMENTS
10.16 OMEGA DIAGNOSTICS GROUP PLC
10.16.1 COMPANY SNAPSHOT
10.16.2 REVENUE ANALYSIS
10.16.3 PRODUCT PORTFOLIO
10.16.4 RECENT DEVELOPMENT
10.17 ORTHO CLINICAL DIAGNOSTICS.
10.17.1 COMPANY SNAPSHOT
10.17.2 REVENUE ANALYSIS
10.17.3 PRODUCT PORTFOLIO
10.17.4 RECENT DEVELOPMENT
10.17.4.1 PRODUCT LAUNCH
10.18 T2 BIOSYSTEM, INC.
10.18.1 COMPANY SNAPSHOT
10.18.2 PRODUCT PORTFOLIO
10.18.3 RECENT DEVELOPMENT
10.18.3.1 PRODUCT LAUNCH
10.19 TRINITY BIOTECH
10.19.1 COMPANY SNAPSHOT
10.19.2 REVENUE ANALYSIS
10.19.3 PRODUCT PORTFOLIO
10.19.4 RECENT DEVELOPMENT
10.2 XCTON DAGNOSTICS LIMITED
10.20.1 COMPANY SNAPSHOT
10.20.2 PRODUCT PORTFOLIO
10.20.3 RECENT DEVELOPMENT
11 QUESTIONNAIRE
12 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 GLOBAL SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 2 GLOBAL IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 3 GLOBAL IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 4 GLOBAL IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 5 GLOBAL MOLECULAR DIAGNOSTICS IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 6 GLOBAL MICROBIOLOGY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 7 GLOBAL FLOW CYTOMETRY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 8 GLOBAL SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 9 GLOBAL LABORATORY TESTING IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 10 GLOBAL POINT OF CARE TESTING IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 11 GLOBAL SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 12 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 14 NORTH AMERICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 15 NORTH AMERICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 16 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 17 U.S. SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 18 U.S. IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 19 U.S. IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 20 U.S. SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 21 REST OF NORTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 22 EUROPE SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 23 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 24 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 25 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 26 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 27 GERMANY SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 28 GERMANY IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 29 GERMANY IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 30 GERMANY SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 31 U.K. SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 32 U.K. IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 33 U.K. IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 34 U.K. SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 35 FRANCE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 36 FRANCE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 37 FRANCE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 38 FRANCE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 39 REST OF EUROPE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 43 ASIA-PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 44 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 45 CHINA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 46 CHINA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 47 CHINA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 48 CHINA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 49 INDIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 50 INDIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 51 INDIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 52 INDIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 53 JAPAN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 54 JAPAN IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 55 JAPAN IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 56 JAPAN SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 57 SOUTH KOREA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 58 SOUTH KOREA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 59 SOUTH KOREA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 60 SOUTH KOREA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 61 AUSTRALIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 62 AUSTRALIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 63 AUSTRALIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 64 AUSTRALIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 65 SINGAPORE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 66 SINGAPORE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 67 SINGAPORE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 68 SINGAPORE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 69 THAILAND SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 70 THAILAND IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 71 THAILAND IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 72 THAILAND SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 73 MALAYSIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 74 MALAYSIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 75 MALAYSIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 76 MALAYSIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 77 INDONESIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 78 INDONESIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 79 INDONESIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 80 INDONESIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 81 PHILIPPINES SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 82 PHILIPPINES IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 83 PHILIPPINES IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 84 PHILIPPINES SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 85 REST OF ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 86 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 87 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 88 SOUTH AMERICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 89 SOUTH AMERICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 90 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 91 BRAZIL SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 92 BRAZIL IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 93 BRAZIL IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 94 BRAZIL SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 95 REST OF SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 96 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 97 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 98 MIDDLE EAST AND AFRICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 99 MIDDLE EAST AND AFRICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 100 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 101 SOUTH AFRICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 102 SOUTH AFRICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 103 SOUTH AFRICA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 104 SOUTH AFRICA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 105 SAUDI ARABIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 106 SAUDI ARABIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 107 SAUDI ARABIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 108 SAUDI ARABIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 109 REST OF MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
Abbildungsverzeichnis
FIGURE 1 GLOBAL SEPSIS DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 GLOBAL SEPSIS DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL SEPSIS DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL SEPSIS DIAGNOSTICS MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL SEPSIS DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL SEPSIS DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL SEPSIS DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 GLOBAL SEPSIS DIAGNOSTICS MARKET: MARKET TECHNIQUES COVERAGE GRID
FIGURE 9 GLOBAL SEPSIS DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL SEPSIS DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF SEPSIS IS EXPECTED TO DRIVE THE GLOBAL SEPSIS DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 TECHNIQUES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL SEPSIS DIAGNOSTICS MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA TO DOMINATE AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE GLOBAL SEPSIS DIAGNOSTICS MARKET FROM 2022 TO 2029
FIGURE 14 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR SEPSIS DIAGNOSTICS MARKET MANUFACTURERS IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 15 DRIVERS, RESTRAINT, OPPORTUNITIES, AND CHALLENGES FOR THE GLOBAL SEPSIS DIAGNOSTICS MARKET
FIGURE 16 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, 2021
FIGURE 17 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, 2022-2029 (USD MILLION)
FIGURE 18 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, CAGR (2022-2029)
FIGURE 19 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, LIFELINE CURVE
FIGURE 20 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, 2021
FIGURE 21 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 22 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 23 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 24 GLOBAL SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 25 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY REGION (2021)
FIGURE 26 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY REGION (2022 & 2029)
FIGURE 27 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY REGION (2021 & 2029)
FIGURE 28 GLOBAL SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 29 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 30 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 31 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 32 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 33 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 34 EUROPE SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 35 EUROPE SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 36 EUROPE SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 37 EUROPE SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 38 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 39 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 40 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 41 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 42 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 43 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 44 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 45 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 46 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 47 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 48 SOUTH AMERICA SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 49 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 50 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 51 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 52 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 53 MIDDLE EAST AND AFRICA SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 54 GLOBAL SEPSIS DIAGNOSTICS MARKET: COMPANY SHARE 2021 (%)
FIGURE 55 NORTH AMERICA SEPSIS DIAGNOSTICS MARKET: COMPANY SHARE 2021 (%)
FIGURE 56 EUROPE SEPSIS DIAGNOSTICS MARKET: COMPANY SHARE 2021 (%)
FIGURE 57 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: COMPANY SHARE 2021 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.