Global Enteral Stents Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
2.39 Billion
USD
3.89 Billion
2024
2032
USD
2.39 Billion
USD
3.89 Billion
2024
2032
| 2025 –2032 | |
| USD 2.39 Billion | |
| USD 3.89 Billion | |
|
|
|
|
Globale Marktsegmentierung für enterale Stents nach Stenttyp (Ösophagusstents, Gastroduodenalstents und Dickdarmstents), Produkttyp (selbstexpandierbare Metallstents (SEMS) und selbstexpandierbare Kunststoffstents (SEPS)), Endverbraucher (Krankenhäuser, Fachkliniken, ambulante Zentren und Forschungszentren) – Branchentrends und Prognose bis 2032
Marktanalyse für enterale Stents
Auf dem Markt für enterale Stents wurden erhebliche Fortschritte bei Materialien, Design und Verabreichungstechniken erzielt. Zu den jüngsten Innovationen gehört die Entwicklung biokompatibler, selbstexpandierender Metallstents aus modernen Legierungen, die Flexibilität, Festigkeit und Biokompatibilität verbessern. Diese Stents sind so konzipiert, dass sie Verstopfungen und Migrationen widerstehen und so zu besseren Patientenergebnissen führen.
Auch die Einführung biologisch abbaubarer enteraler Stents ist ein großer Fortschritt. Diese Stents bauen sich nach Erfüllung ihres Zwecks auf natürliche Weise ab, wodurch die Notwendigkeit von Entfernungsverfahren verringert und das Risiko langfristiger Komplikationen minimiert wird. Darüber hinaus ermöglichen Fortschritte in der Bildgebungstechnologie, wie beispielsweise endoskopische und fluoroskopische Führung, eine präzisere Platzierung der Stents und verbessern so ihre Wirksamkeit.
Das Wachstum des Marktes für enterale Stents wird durch die steigende Prävalenz von Magen-Darm-Erkrankungen wie Speiseröhrenkrebs und das zunehmende Bewusstsein für minimalinvasive Behandlungen vorangetrieben. Da die Bevölkerung immer älter wird und Gesundheitsdienstleister sich auf die Verbesserung der Lebensqualität der Patienten konzentrieren, steigt die Nachfrage nach enteralen Stents weiter an. Verbesserte Technologie und bessere Behandlungsergebnisse werden in den kommenden Jahren voraussichtlich zu weiterem Marktwachstum führen.
Marktgröße für enterale Stents
Der weltweite Markt für enterale Stents wurde im Jahr 2024 auf 2,39 Milliarden US-Dollar geschätzt und soll bis 2032 3,89 Milliarden US-Dollar erreichen, mit einer durchschnittlichen jährlichen Wachstumsrate von 6,28 % im Prognosezeitraum von 2025 bis 2032. Neben Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research zusammengestellten Marktberichte auch eingehende Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
Markttrends für enterale Stents
„Steigende Nachfrage nach minimalinvasiven Eingriffen“
Ein wichtiger Trend, der das Wachstum des Marktes für enterale Stents vorantreibt, ist die zunehmende Präferenz für minimalinvasive Verfahren. Enterale Stents werden zur Behandlung von Magen-Darm-Blockaden eingesetzt und sind unerlässlich, um Linderung zu verschaffen, ohne dass eine umfangreiche Operation erforderlich ist. Das wachsende Bewusstsein für die Vorteile dieser Verfahren, darunter kürzere Genesungszeiten und kürzere Krankenhausaufenthalte, treibt die Marktnachfrage an. Beispielsweise entscheiden sich Krankenhäuser und Kliniken weltweit für die Platzierung von Stents anstelle von chirurgischen Bypass-Verfahren, da Stents schnellere und weniger traumatische Optionen für Patienten mit Erkrankungen wie Speiseröhrenkrebs oder Morbus Crohn bieten. Dieser Wechsel hin zu minimalinvasiven Behandlungen dürfte die Einführung enteraler Stents beschleunigen und erheblich zur Marktexpansion beitragen.
Berichtsumfang und Marktsegmentierung für enterale Stents
|
Eigenschaften |
Wichtige Markteinblicke zu enteralen Stents |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
USA, Kanada und Mexiko in Nordamerika, Deutschland, Frankreich, Großbritannien, Niederlande, Schweiz, Belgien, Russland, Italien, Spanien, Türkei, Restliches Europa in Europa, China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, Philippinen, Restlicher Asien-Pazifik-Raum (APAC) in Asien-Pazifik (APAC), Saudi-Arabien, Vereinigte Arabische Emirate, Südafrika, Ägypten, Israel, Restlicher Naher Osten und Afrika (MEA) als Teil von Naher Osten und Afrika (MEA), Brasilien, Argentinien und Restliches Südamerika als Teil von Südamerika |
|
Wichtige Marktteilnehmer |
Boston Scientific Corporation (USA), Cook (USA), Abbott (USA), Biosensors International Group, Ltd. (Singapur), Stryker (USA), Medtronic (USA), B. Braun SE (Deutschland), BD (USA), Cardinal Health (USA), Terumo Europe NV (Belgien), TAEWOONG (Südkorea), ELLA - CS, sro (Tschechische Republik), Merit Medical Systems (USA), MITECH. (Südkorea), BVM Medical Limited (Großbritannien), Changzhou Health Microport Medical Device Co., LTD. (China), ENDO-FLEX GmbH (Deutschland), Micro-Tech (Nanjing) Co., Ltd. (China) und GBUK Group Ltd. (Großbritannien) |
|
Marktchancen |
|
|
Wertschöpfende Dateninfosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research zusammengestellten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen. |
Enterale Stents Marktdefinition
Enterale Stents sind medizinische Geräte zur Behandlung von Blockaden oder Verengungen im Verdauungstrakt, einschließlich der Speiseröhre, des Zwölffingerdarms oder des Dickdarms. Sie bestehen normalerweise aus Materialien wie Metall oder Silikon und werden in den betroffenen Bereich eingeführt, um den Durchgang offen zu halten. Dies hilft, den Fluss von Nahrung, Flüssigkeiten oder Abfallstoffen aufrechtzuerhalten und verbessert die Fähigkeit des Patienten zu essen und zu verdauen. Enterale Stents werden häufig bei Patienten mit Erkrankungen wie Krebs, Morbus Crohn oder anderen Magen-Darm-Erkrankungen verwendet, da sie eine weniger invasive Alternative zu chirurgischen Eingriffen darstellen und die allgemeine Lebensqualität verbessern.
Marktdynamik für enterale Stents
Treiber
- Zunehmende Prävalenz von Magen-Darm-Erkrankungen
Die zunehmende Verbreitung von Magen-Darm-Erkrankungen wie Dickdarmkrebs, Speiseröhrenkrebs und Morbus Crohn ist ein wichtiger Treiber des Marktes für enterale Stents. Diese Erkrankungen führen häufig zu Verstopfungen im Verdauungstrakt, die Eingriffe erfordern, um den ordnungsgemäßen Durchgang für Nahrung und Flüssigkeiten wiederherzustellen. Bei Patienten mit Speiseröhrenkrebs beispielsweise, bei denen Tumore die Speiseröhre blockieren können, werden enterale Stents verwendet, um einen freien Durchgang zum Schlucken aufrechtzuerhalten und so die Lebensqualität der Patienten zu verbessern. Ebenso werden bei Dickdarmkrebs oder Morbus Crohn Stents verwendet, um Symptome zu lindern und chirurgische Eingriffe zu vermeiden. Da diese Erkrankungen weltweit zunehmen, wächst die Nachfrage nach enteralen Stents als nicht-chirurgische Lösung weiter, was die Marktexpansion fördert.
- Steigende Nachfrage nach Palliativversorgung
Der wachsende Fokus auf Palliativmedizin, insbesondere in der Onkologie, treibt den Markt für enterale Stents an. Enterale Stents werden häufig zur Behandlung von Symptomen bei Krebspatienten im Endstadium eingesetzt, beispielsweise zur Linderung von Speiseröhren- oder Magen-Darm-Verschlüssen. Dieser palliative Ansatz trägt dazu bei, die Lebensqualität von Patienten zu verbessern, für die eine kurative Operation nicht in Frage kommt. Bei Patienten mit fortgeschrittenem Speiseröhrenkrebs werden beispielsweise häufig selbstexpandierende Metallstents verwendet, um die Schluckfunktion wiederherzustellen und so eine Linderung der Symptome zu erzielen. Die zunehmende Anerkennung der Bedeutung der Palliativmedizin in der Krebsbehandlung sowie die alternde Bevölkerung und die steigenden Krebsraten tragen erheblich zur steigenden Nachfrage nach enteralen Stents in diesem Bereich bei.
Gelegenheiten
- Fortschritte bei Stentmaterialien
Die Entwicklung biokompatibler und langlebiger Materialien hat dem Markt für enterale Stents erhebliche Chancen eröffnet. Innovationen wie selbstexpandierende Metallstents (SEMS) aus Legierungen wie Nitinol bieten überlegene Flexibilität, Haltbarkeit und Korrosionsbeständigkeit. Darüber hinaus bietet die Entwicklung biologisch abbaubarer Stents aus Materialien wie Polymilchsäure (PLA) eine vorübergehende Lösung, wodurch die Notwendigkeit von Entfernungsverfahren reduziert wird. Diese Fortschritte verbessern nicht nur die Wirksamkeit von Stents, sondern minimieren auch Komplikationen wie Infektionen und Verstopfungen. Beispielsweise hat die Verwendung beschichteter Stents bei der Behandlung von Speiseröhrenkrebs die Ergebnisse verbessert, indem das Einwachsen und die Migration von Tumoren reduziert wurden, wodurch die Marktnachfrage nach derartigen fortschrittlichen Stenttechnologien steigt.
- Regierungsinitiativen zur Krebsbehandlung
Regierungsinitiativen zur Verbesserung der Krebsbehandlung schaffen erhebliche Chancen auf dem Markt für enterale Stents. Mit zunehmendem Fokus auf bessere Behandlungsmöglichkeiten finanzieren viele Regierungen fortschrittliche Therapien für Krebspatienten, darunter minimalinvasive Lösungen wie enterale Stents. Laut der Weltgesundheitsorganisation 2023 ist beispielsweise Dickdarmkrebs die dritthäufigste Krebsart, die für etwa 10 % aller Fälle verantwortlich ist, und die zweithäufigste krebsbedingte Todesursache weltweit. Das Global Cancer Observatory meldete im Jahr 2020 fast 1,15 Millionen Neuerkrankungen, und Prognosen gehen davon aus, dass es bis 2040 weltweit etwa 1,92 Millionen Neuerkrankungen geben wird.
Einschränkungen/Herausforderungen
- Kosten- und Erstattungsfragen
Kosten- und Erstattungsprobleme behindern den Markt für enterale Stents erheblich. Die hohen Kosten dieser Geräte können ein großes Hindernis darstellen, insbesondere in Gesundheitssystemen mit begrenzten Budgets oder preisempfindlichen Bevölkerungsgruppen. In vielen Regionen sind die Erstattungsrichtlinien für enterale Stents restriktiv oder unzureichend, was dazu führt, dass sich Patienten den Eingriff nicht leisten können. Gesundheitssysteme decken die Kosten möglicherweise nicht vollständig ab, sodass Patienten die Kosten entweder selbst tragen oder ganz auf die Behandlung verzichten müssen. Dies führt zu geringeren Akzeptanzraten und begrenzt das Wachstumspotenzial des Marktes. Darüber hinaus zögern Krankenhäuser und Gesundheitsdienstleister möglicherweise, in teure Stents zu investieren, wenn sie keine ausreichende finanzielle Unterstützung oder Erstattungsgarantien haben.
- Komplikationswahrscheinlichkeit und Risiken
Die mit enteralen Stents verbundenen Komplikationswahrscheinlichkeiten und Risiken behindern das Marktwachstum erheblich. Migration von Stents, Perforation des Magen-Darm-Trakts und Infektionen sind häufige Probleme, die nach der Platzierung von Stents auftreten können. Diese Komplikationen erfordern oft zusätzliche medizinische Eingriffe, was zu längeren Krankenhausaufenthalten und höheren Gesundheitskosten führt. Darüber hinaus können die Notwendigkeit von Nachbehandlungen oder die Entfernung der Stents zu Beschwerden des Patienten beitragen und die Genesungszeit verlängern. Solche Risiken halten sowohl Gesundheitsdienstleister als auch Patienten davon ab, sich für enterale Stents zu entscheiden, was zu einem Rückgang der Akzeptanzraten führt. Infolgedessen stellen diese Komplikationen eine erhebliche Herausforderung für das Wachstum des Marktes für enterale Stents dar.
Dieser Marktbericht enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-Export-Analysen, Produktionsanalysen, Wertschöpfungskettenoptimierungen, Marktanteilen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neue Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Markt zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analystenbericht zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Marktumfang für enterale Stents
Der Markt ist nach Produkttyp, Stenttyp und Endbenutzer segmentiert. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, die ihnen bei der strategischen Entscheidungsfindung zur Identifizierung der wichtigsten Marktanwendungen helfen.
Stenttyp
- Ösophagusstents
- Gastroduodenale Stents
- Kolonstents
- Präoperative Dekompression
- Linderung fortgeschrittener maligner Erkrankungen
Produkttyp
- Selbstexpandierbare Metallstents (SEMS)
- Unbedeckte SEMS
- Teilweise abgedeckte SEMS
- Vollständig abgedeckte SEMS
- Selbstexpandierbare Kunststoffstents (SEPS)
Endbenutzer
- Krankenhäuser
- Spezialkliniken
- Ambulante Zentren
- Forschungszentren
Regionale Analyse des enteralen Stents-Marktes
Der Markt wird analysiert und es werden Einblicke in die Marktgröße und Trends nach Land, Produkttyp, Stenttyp und Endbenutzer wie oben angegeben bereitgestellt.
Die im Marktbericht abgedeckten Länder sind die USA, Kanada, Mexiko in Nordamerika, Deutschland, Schweden, Polen, Dänemark, Italien, Großbritannien, Frankreich, Spanien, Niederlande, Belgien, Schweiz, Türkei, Russland, Restliches Europa in Europa, Japan, China, Indien, Südkorea, Neuseeland, Vietnam, Australien, Singapur, Malaysia, Thailand, Indonesien, Philippinen, Restlicher Asien-Pazifik-Raum (APAC) in Asien-Pazifik (APAC), Brasilien, Argentinien, Restliches Südamerika als Teil von Südamerika, Vereinigte Arabische Emirate, Saudi-Arabien, Oman, Katar, Kuwait, Südafrika, Restlicher Naher Osten und Afrika (MEA) als Teil von Naher Osten und Afrika (MEA)
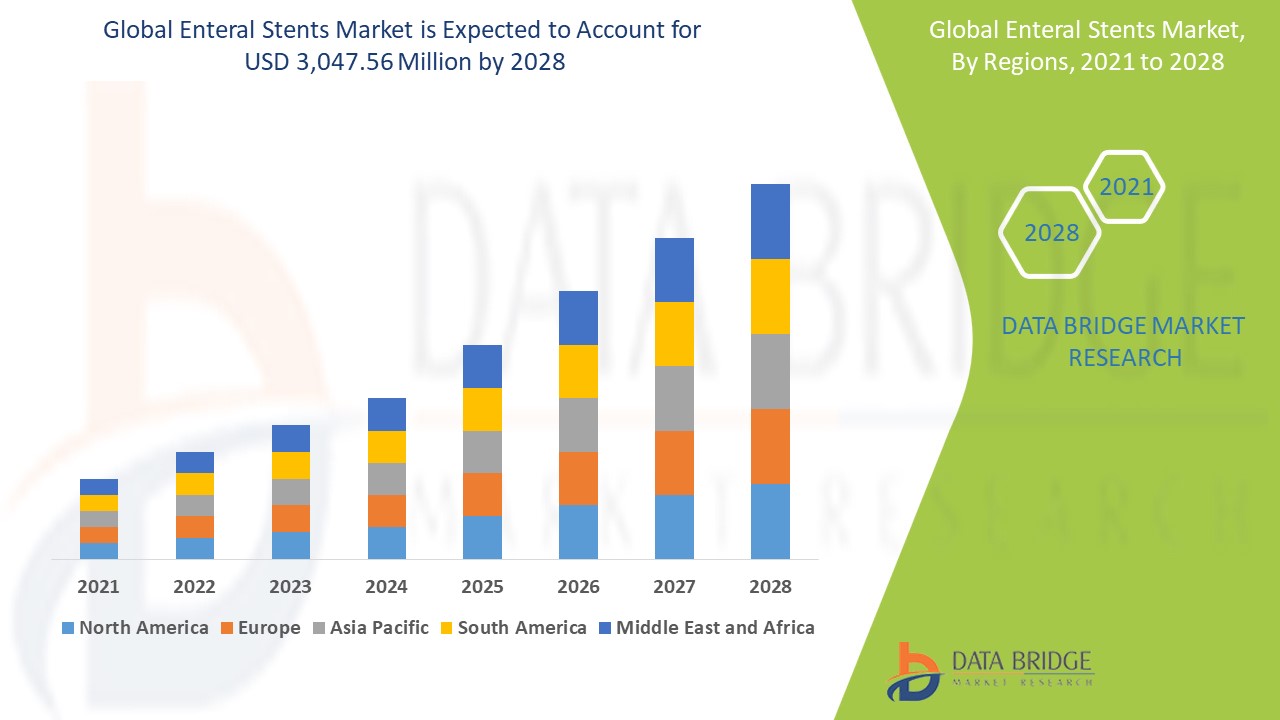
North America is expected to dominate the enteral stents market due to technological advancements, a rising prevalence of cancer, and lifestyle changes in the region. The growing demand for minimally invasive procedures and improved healthcare infrastructure further boosts the adoption of enteral stents, positioning North America as the leading market player.
Asia-Pacific is expected to grow at the highest growth rate in the enteral stents market during the forecast period. This growth is driven by advancements in healthcare infrastructure, increasing per capita healthcare expenditure, and rising demand for minimally invasive procedures, particularly in countries such as China and India.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Enteral Stents Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Enteral Stents Market Leaders Operating in the Market Are:
- Boston Scientific Corporation (U.S.)
- Cook (U.S.)
- Abbott (U.S.)
- Biosensors International Group, Ltd. (Singapore)
- Stryker (U.S.)
- Medtronic (U.S.)
- B. Braun SE (Germany)
- BD (U.S.)
- Cardinal Health (U.S.)
- Terumo Europe NV (Belgium)
- TAEWOONG (South Korea)
- ELLA - CS, s.r.o. (Czech Republic)
- Merit Medical Systems (U.S.)
- M.I.TECH. (South Korea)
- BVM Medical Limited (U.K.)
- Changzhou Health Microport Medical Device Co., LTD. (China)
- ENDO-FLEX GmbH (Germany)
- Micro-Tech (Nanjing) Co., Ltd. (China)
- GBUK Group Ltd. (U.K.)
Latest Developments in Enteral Stents Market
- In November 2022, The Union Health Ministry of India announced the inclusion of coronary stents in the National List of Essential Medicines 2022. This decision was aimed at making coronary stents more affordable for the Indian population, thereby improving access to life-saving treatments for heart conditions and enhancing overall cardiovascular care across the country
- Im Mai 2022 erhielt Medtronic von der US-amerikanischen FDA die Zulassung für den medikamentenfreisetzenden Stent Onyx Frontier (DES), einen bedeutenden Fortschritt in der Herz-Kreislauf-Behandlung. Dieser Stent verfügt über ein einzigartiges Design, das die Sicherheit und Wirksamkeit von Herzoperationen verbessert und Patienten mit koronarer Herzkrankheit verbesserte klinische Ergebnisse bietet, wobei der Schwerpunkt auf der langfristigen Leistung liegt
- Im April 2022 brachte Translumina, ein führender Hersteller von kardiovaskulären Medizinprodukten, seinen innovativen VIVO ISAR-Stent (DDCS) mit dualer Wirkstofffreisetzung und Polymerbeschichtung auf internationale Märkte, darunter auch Europa. Der Stent bietet eine verbesserte Wirkstoffverabreichung und Gefäßheilung, stellt eine Alternative zu herkömmlichen medikamentenfreisetzenden Stents dar und verbessert die Behandlungsmöglichkeiten für Patienten, die sich weltweit interventionellen kardiologischen Eingriffen unterziehen.
- Im Oktober 2020 brachte Olympus, ein führendes Unternehmen im Bereich der Medizintechnik, die selbstexpandierenden Metallstents (SEMS) HANAROSTENT Esophagus TTS in den USA auf den Markt. Diese von MI Tech hergestellten und exklusiv über Olympus vertriebenen Stents sind für die Behandlung von Erkrankungen der Speiseröhre konzipiert und bieten Ärzten eine zuverlässige Lösung zur Behandlung obstruktiver und bösartiger Erkrankungen der Speiseröhre, wodurch die Patientenversorgung und die Lebensqualität verbessert werden.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.