Global Dengue Vaccine Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
661.27 Million
USD
1,602.53 Million
2024
2032
USD
661.27 Million
USD
1,602.53 Million
2024
2032
| 2025 –2032 | |
| USD 661.27 Million | |
| USD 1,602.53 Million | |
|
|
|
|
Globale Marktsegmentierung für Dengue-Impfstoffe nach Typ ( abgeschwächter Lebendimpfstoff , chimärer abgeschwächter Lebendimpfstoff, inaktivierter Impfstoff, Untereinheitenimpfstoff und nukleinsäurebasierter Impfstoff), Behandlung (Diuretikum, Antiallergikum, Blutverdünner und andere), Verabreichungsweg (oral, parenteral und andere), Endverbraucher (Krankenhäuser, häusliche Pflege, Fachkliniken und andere), Vertriebskanal (Krankenhausapotheke, Online-Apotheke und Einzelhandelsapotheke) – Branchentrends und Prognose bis 2032
Dengue-Impfstoff Marktgröße
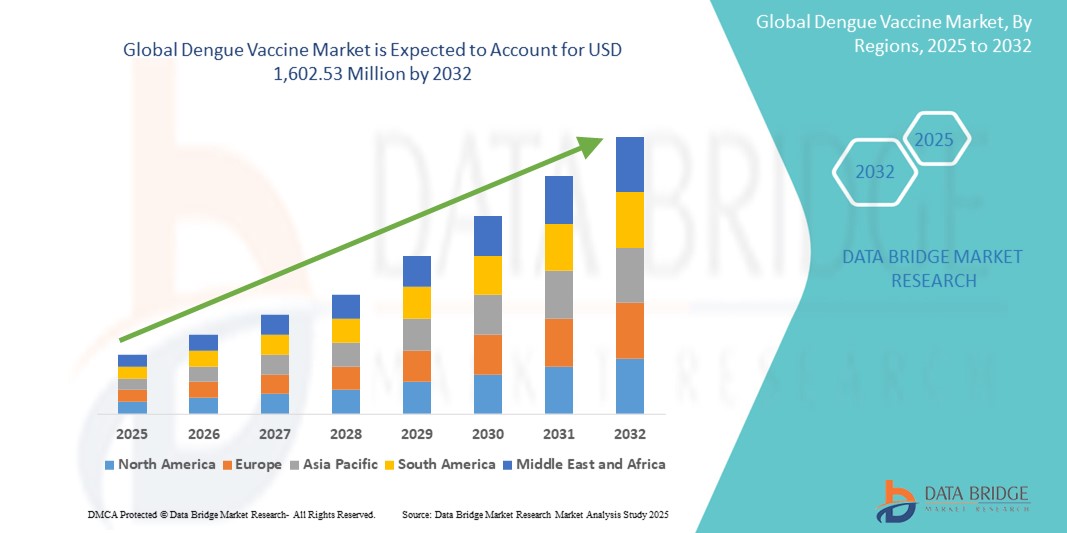
- Der globale Markt für Dengue-Impfstoffe wurde im Jahr 2024 auf 661,27 Millionen US-Dollar geschätzt und dürfte bis 2032 einen Wert von 1.602,53 Millionen US-Dollar erreichen , was einer jährlichen Wachstumsrate (CAGR) von 11,70 % im Prognosezeitraum entspricht.
- Das Marktwachstum wird maßgeblich durch die weltweit steigende Verbreitung des Denguefiebers, das zunehmende Gesundheitsbewusstsein der Bevölkerung und staatliche Impfinitiativen zur Bekämpfung von durch Mücken übertragenen Krankheiten vorangetrieben, insbesondere in endemischen Regionen wie Südostasien, Lateinamerika und Teilen Afrikas.
- Darüber hinaus macht die steigende Nachfrage nach sicheren, wirksamen und langfristigen Immunisierungslösungen Dengue-Impfstoffe zu einem wichtigen Bestandteil öffentlicher Gesundheitsstrategien. Diese zusammenlaufenden Faktoren beschleunigen die Akzeptanz von Dengue-Impfstofflösungen und kurbeln damit das Wachstum des Dengue-Impfstoffmarktes deutlich an.
Marktanalyse für Dengue-Impfstoffe
- Dengue-Impfstoffe, die vor dem von Mücken übertragenen Dengue-Virus schützen sollen, gewinnen aufgrund der zunehmenden Zahl von Dengue-Infektionen in tropischen und subtropischen Regionen zunehmend an Bedeutung als wichtiges Instrument der öffentlichen Gesundheit. Die weltweit zunehmende Belastung durch Denguefieber und die Bemühungen, Morbidität und Mortalität zu senken, treiben die Nachfrage nach wirksamen und zugänglichen Dengue-Impfprogrammen voran.
- Das wachsende Bewusstsein für die gesundheitlichen Auswirkungen von Dengue-Fieber, verbunden mit unterstützenden staatlichen Impfinitiativen und der wachsenden Gesundheitsinfrastruktur in endemischen Regionen, hat das Marktwachstum erheblich angekurbelt.
- Nordamerika dominierte den Markt für Dengue-Impfstoffe mit dem größten Umsatzanteil von 46,0 % im Jahr 2024, was auf starke Investitionen in Forschung und Entwicklung, frühzeitige behördliche Zulassungen und steigende Impfanforderungen für Reisende zurückzuführen ist. Insbesondere die USA verzeichneten ein erhebliches Marktwachstum durch die Einführung von Dengue-Impfstoffen für Reisende und Militärpersonal, das in Dengue-Endemiegebieten stationiert ist.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum mit einer prognostizierten jährlichen Wachstumsrate von 23,6 % die am schnellsten wachsende Region im Dengue-Impfstoffmarkt sein. Dieses Wachstum ist auf die hohe Prävalenz von Dengue-Fällen in Ländern wie Indien, Indonesien, den Philippinen und Thailand sowie auf die zunehmende staatliche Unterstützung von Massenimpfkampagnen und die verbesserte Verfügbarkeit von Impfstoffen zurückzuführen.
- Das parenterale Segment dominierte den Markt für Dengue-Impfstoffe mit einem Marktanteil von 67,5 % im Jahr 2024 , da die meisten derzeit verfügbaren Dengue-Impfstoffe durch Injektion verabreicht werden und ärztlicher Überwachung bedürfen. Diese Methode gewährleistet eine genaue Dosierung und Wirksamkeit, insbesondere bei Impfprogrammen von Gesundheitseinrichtungen in endemischen Regionen.
Berichtsumfang und Marktsegmentierung für Dengue-Impfstoffe
|
Eigenschaften |
Wichtige Markteinblicke zum Dengue-Impfstoff |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für Dengue-Impfstoffe
„ Wachsendes öffentliches Gesundheitsbewusstsein und Initiativen zur Gesundheitsvorsorge “
- Ein bedeutender und sich beschleunigender Trend auf dem globalen Dengue-Impfstoffmarkt ist das zunehmende öffentliche Bewusstsein für durch Mücken übertragene Krankheiten und die Bedeutung der Impfung als vorbeugende Maßnahme. Dieses steigende Gesundheitsbewusstsein, insbesondere in endemischen Regionen, treibt die Nachfrage nach wirksamen und lang anhaltenden Dengue-Impfstoffen an.
- Beispielsweise tragen staatlich geförderte Kampagnen und Initiativen globaler Gesundheitsorganisationen wie der Weltgesundheitsorganisation (WHO) und der Impfallianz GAVI dazu bei, die Impfabdeckung in Hochrisikoregionen wie Südostasien, Lateinamerika und Afrika zu verbessern. Diese Bemühungen tragen erheblich dazu bei, das öffentliche Verständnis für die Vorteile der Dengue-Impfung zu stärken.
- Impfstoffhersteller konzentrieren sich zunehmend auf die Entwicklung multivalenter Formulierungen, die alle vier Dengue-Virus-Serotypen abdecken. Der Erfolg des QDENGA-Impfstoffs von Takeda bei der Zulassung in Ländern wie Indonesien und Brasilien spiegelt diesen Trend hin zu umfassenderen Immunisierungslösungen wider.
- Die zunehmende Beteiligung sowohl des öffentlichen als auch des privaten Gesundheitssektors an der Einführung von Dengue-Impfprogrammen hat auch zu Verbesserungen der Kühlketteninfrastruktur, der Impfstofflagerlogistik und der Zustellung auf der letzten Meile geführt – entscheidende Faktoren für die Gewährleistung der Impfstoffwirksamkeit in der ländlichen und städtischen Bevölkerung.
- Darüber hinaus entsteht durch die zunehmende Zahl internationaler Reisen in Dengue-Endemiegebiete ein Markt für Reiseimpfungen, der eine Nischennachfrage nach Dengue-Impfungen vor der Reise schafft. Kliniken und Apotheken, die reisebezogene Impfungen anbieten, erweitern ihr Angebot um Dengue-Impfstoffe, insbesondere in Nordamerika und Europa.
- Dieser Trend zu präventiver Gesundheitsfürsorge und bevölkerungsweiter Immunisierung verändert die globalen Strategien zur Bekämpfung von Vektorkrankheiten grundlegend. Da die Länder zunehmend in Maßnahmen zur Dengue-Kontrolle investieren, wird die Nachfrage nach sicheren und wirksamen Impfstoffen voraussichtlich deutlich steigen. Dies bietet Pharmaentwicklern und Gesundheitsbehörden langfristige Chancen.
Marktdynamik für Dengue-Impfstoffe
Treiber
„Steigender Bedarf aufgrund der zunehmenden weltweiten Dengue-Belastung und der Einführung von Impfstoffen“
- Die zunehmende Verbreitung von Denguefieber, insbesondere in tropischen und subtropischen Regionen, sowie Klimawandel und Urbanisierung treiben die Nachfrage nach Dengue-Impfstoffen deutlich an. Regierungen und globale Gesundheitsorganisationen beschleunigen Impfprogramme, um Ausbrüche zu verhindern und die Belastung des Gesundheitswesens zu verringern.
- So wurde beispielsweise im Mai 2024 Takedas Dengue-Impfstoff QDENGA nach vielversprechenden Ergebnissen klinischer Studien in Brasilien und Indonesien in die erweiterten Impfpläne aufgenommen. Solche Strategien wichtiger Unternehmen dürften das Wachstum der Dengue-Impfstoffbranche im Prognosezeitraum vorantreiben.
- Da das Bewusstsein für die Dengue-Prävention wächst und der Zugang zu Impfungen verbessert wird, entscheiden sich immer mehr Menschen für eine Impfung gegen die Krankheit. Die praktische Anwendung präventiver Impfungen, insbesondere für Risikogruppen wie Reisende, Kinder und medizinisches Personal, fördert die Marktakzeptanz zusätzlich.
- Darüber hinaus sorgen Kooperationen zwischen internationalen Organisationen wie der WHO und Gavi sowie die Aufnahme von Dengue-Impfstoffen in nationale Impfprogramme dafür, dass diese Impfstoffe insbesondere in Ländern mit niedrigem und mittlerem Einkommen leichter zugänglich sind.
- Die verstärkte Überwachung von durch Vektoren übertragenen Krankheiten, die Unterstützung von Initiativen in endemischen Ländern und die laufende Entwicklung von Impfstoffen der nächsten Generation, die auf mehrere Serotypen abzielen, tragen zum langfristigen Marktwachstum bei
Einschränkung/Herausforderung
„ Bedenken hinsichtlich der Impfstoffsicherheit und der hohen Entwicklungskosten “
- Bedenken hinsichtlich der Impfstoffsicherheit, insbesondere bei Personen ohne vorherige Dengue-Exposition, stellen eine erhebliche Herausforderung für eine breitere Marktakzeptanz dar. Frühere Kontroversen, wie beispielsweise um Sanofis Dengvaxia auf den Philippinen, haben sowohl Gesundheitsdienstleister als auch die Öffentlichkeit vorsichtig gemacht.
- So betont die WHO beispielsweise weiterhin die Notwendigkeit eines Serostatustests vor der Verabreichung von Dengvaxia aufgrund der Risiken bei seronegativen Personen, was den großflächigen Einsatz einschränkt und die Logistik der Einführung erschwert.
- Um das Vertrauen der Öffentlichkeit wiederherzustellen, ist es entscheidend, diese Bedenken durch verbesserte Diagnoseinstrumente, reale Sicherheitsdaten und transparente Kommunikation auszuräumen. Neuere Impfstoffe wie QDENGA gelten als sicherere Alternativen mit weniger Einschränkungen.
Darüber hinaus können die hohen Kosten für Entwicklung, Herstellung und Vertrieb von Impfstoffen – insbesondere bei multivalenten Impfstoffen – ein Hindernis für die Einführung darstellen, insbesondere in ressourcenarmen Regionen. - Während die globale Unterstützung durch Finanzierungsagenturen und Partnerschaften dazu beiträgt, Zugangslücken zu schließen, sind kontinuierliche Investitionen, eine Vereinfachung der Vorschriften und Aufklärungsbemühungen von entscheidender Bedeutung, um diese Herausforderungen zu bewältigen und eine breite Impfstoffabdeckung in endemischen Regionen zu erreichen.
Dengue-Impfstoff Marktumfang
Der Markt ist nach Art, Behandlung, Verabreichungsweg, Endbenutzern und Vertriebskanal segmentiert.
• Nach Typ
Der Markt für Dengue-Impfstoffe ist nach Typ in Lebendimpfstoffe, chimäre Lebendimpfstoffe, inaktivierte Impfstoffe, Untereinheitenimpfstoffe und nukleinsäurebasierte Impfstoffe unterteilt. Das Segment der Lebendimpfstoffe hatte im Jahr 2024 mit 42,3 % den größten Marktanteil, was auf seine breite Akzeptanz und Verwendung in endemischen Regionen zurückzuführen ist.
Im Segment der Impfstoffe auf Nukleinsäurebasis wird von 2025 bis 2032 voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate (CAGR) von 22,3 % verzeichnet, was auf die zunehmende Verbreitung von mRNA- und DNA-Plattformen zurückzuführen ist, die eine schnelle Entwicklung und Skalierbarkeit ermöglichen.
• Durch Behandlung
Der Markt für Dengue-Impfstoffe ist nach Behandlungsmethoden in Diuretika, Antiallergika, Blutverdünner und weitere Mittel unterteilt. Das Segment der Antiallergika hatte im Jahr 2024 mit 38,7 % den größten Marktanteil, was auf seine entscheidende Rolle bei der Symptombehandlung von Hautausschlägen und allergischen Reaktionen zurückzuführen ist.
Für das Segment der Blutverdünner wird von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 18,6 % das höchste Wachstum erwartet, da schwere Dengue-Komplikationen im Zusammenhang mit Gefäß- und Gerinnungsstörungen zunehmend in den Fokus rücken.
• Nach Verabreichungsweg
Der Markt für Dengue-Impfstoffe wird je nach Verabreichungsweg in orale, parenterale und andere Impfstoffe unterteilt. Das parenterale Segment hatte im Jahr 2024 mit 67,5 % den höchsten Umsatzanteil, da die meisten aktuellen Dengue-Impfstoffe injizierbar sind und unter ärztlicher Aufsicht verabreicht werden.
Das orale Segment dürfte zwischen 2025 und 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 19,2 % am schnellsten wachsen, angetrieben durch Innovationen bei oralen Verabreichungsmethoden, insbesondere für die Anwendung in der Pädiatrie und bei Massenimmunisierungen.
• Von Endbenutzern
Der Markt für Dengue-Impfstoffe ist nach Endverbrauchern in Krankenhäuser, häusliche Pflege, Fachkliniken und andere Bereiche unterteilt. Das Krankenhaussegment führte den Markt mit einem Umsatzanteil von 49,3 % im Jahr 2024 an, was auf seine entscheidende Rolle bei der Impfstofflieferung, der Versorgung von Dengue-Patienten und der Lagerung zurückzuführen ist.
Das Segment der Fachkliniken dürfte zwischen 2025 und 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 17,4 % am schnellsten wachsen, da in städtischen Gebieten zunehmend ambulante Impfdienste bevorzugt werden.
• Nach Vertriebskanal
Der Markt für Dengue-Impfstoffe ist nach Vertriebskanälen in Krankenhausapotheken, Online-Apotheken und stationäre Apotheken unterteilt. Das Segment der Krankenhausapotheken dominierte mit einem Marktanteil von 45,8 % im Jahr 2024, unterstützt durch robuste Vertriebsnetze mit Anbindung an Gesundheitseinrichtungen.
Das Segment der Online-Apotheken wird voraussichtlich zwischen 2025 und 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 20,1 % wachsen, angetrieben durch wachsende E-Health-Plattformen und Impfstoff-Lieferdienste bis an die Haustür.
Regionale Analyse des Dengue-Impfstoffmarktes
- Nordamerika dominierte den Markt für Dengue-Impfstoffe mit dem größten Umsatzanteil von 46,0 % im Jahr 2024. Dies ist auf das steigende Bewusstsein für Dengue-Ausbrüche, proaktive Impfstrategien und starke staatliche Unterstützung zurückzuführen. Die Region profitiert von einer fortschrittlichen Gesundheitsinfrastruktur und der frühen Zulassung von Impfstoffen wie TAK-003 (Qdenga) und Dengvaxia, was eine schnelle Verteilung und Akzeptanz in den USA, Mexiko und den karibischen Staaten ermöglicht.
- Die zunehmende Verbreitung von Dengue-Fällen, insbesondere in wärmeren US-Bundesstaaten und reiseintensiven Regionen, hat die Nachfrage nach vorbeugenden Impfungen angekurbelt. Auch öffentliche Gesundheitskampagnen und Kooperationen mit Pharmaunternehmen haben zur regionalen Dominanz beigetragen.
- Diese robuste Marktexpansion wird zusätzlich durch hohe Gesundheitsausgaben, die schnelle Einführung innovativer Impfstofftechnologien und verstärkte Impfprogramme für Reisende in Endemiegebieten unterstützt.
Einblicke in den US -Dengue-Impfstoffmarkt
Der US-Markt für Dengue-Impfstoffe erzielte 2024 mit 71 % den größten Umsatzanteil innerhalb Nordamerikas. Dies ist auf laufende Investitionen in Forschung und Entwicklung, FDA-regulierte Zulassungsverfahren und die wachsende Besorgnis über vektorübertragene Krankheiten aufgrund des Klimawandels zurückzuführen. Der Markt wird zusätzlich durch die starke biopharmazeutische Präsenz des Landes und die hohe Nachfrage nach Reiseimpfstoffen, insbesondere bei Militärangehörigen und Touristen, angetrieben. Darüber hinaus besteht in den USA weiterhin ein erhöhtes Interesse an klinischen Studien und öffentlich-privaten Kooperationen zur Stärkung der Impfstoffpipelines.
Einblicke in den europäischen Dengue-Impfstoffmarkt
Der europäische Markt für Dengue-Impfstoffe wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen. Dies ist auf das gestiegene Bewusstsein für Dengue-Prävention zurückzuführen, insbesondere bei Reisenden, Migranten und der Bevölkerung in Überseegebieten. Die strenge Gesundheitspolitik der Region, reisemedizinische Initiativen und der Fokus auf das Management tropischer Krankheiten treiben die Impfstoffnachfrage an. Länder wie Frankreich, Deutschland und Großbritannien leisten wichtige Beiträge und beteiligen sich zunehmend an internationalen Dengue-Impfstoff-Forschungsprogrammen.
Einblicke in den britischen Dengue-Impfstoffmarkt
Der britische Markt für Dengue-Impfstoffe wird im Prognosezeitraum voraussichtlich mit einer bemerkenswerten jährlichen Wachstumsrate wachsen, unterstützt durch eine starke globale Reisekultur und die steigende Nachfrage nach Impfungen vor Reisen. Aufklärungskampagnen im Bereich der öffentlichen Gesundheit sowie Reiseimpfprogramme des NHS erhöhen die Akzeptanz von Dengue-Impfstoffen. Großbritannien engagiert sich zudem aktiv in globalen Gesundheitspartnerschaften, die den Zugang zu Impfstoffen in Endemiegebieten fördern und so indirekt das lokale Wachstum durch Produktions- und Exportpartnerschaften unterstützen.
Markteinblicke für Dengue-Impfstoffe in Deutschland
Der deutsche Dengue-Impfstoffmarkt wird im Prognosezeitraum voraussichtlich mit einer beträchtlichen jährlichen Wachstumsrate wachsen. Dies ist auf die starke Beteiligung an der internationalen Impfstoffentwicklung, staatlich geförderte Gesundheitsaufklärungsprogramme und hohe Gesundheitsausgaben zurückzuführen. Deutschlands Schwerpunkt auf digitale Gesundheit und Innovation ermöglicht zudem eine effektive Verfolgung von Dengue-Fällen und verbessert so die risikobasierte Impfstoffverteilung im In- und Ausland.
Einblicke in den Dengue-Impfstoffmarkt im asiatisch-pazifischen Raum
Der Markt für Dengue-Impfstoffe im asiatisch-pazifischen Raum dürfte von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 23,6 % die höchste Wachstumsrate aufweisen. Die Region weist weltweit die höchste Dengue-Belastung auf, insbesondere in Ländern wie Indien, Indonesien, Thailand und den Philippinen. Staatlich geförderte Massenimpfkampagnen, von der WHO geleitete Aufklärungsinitiativen und die rasante Urbanisierung tragen maßgeblich zum Marktwachstum bei. Die Präsenz einheimischer Impfstoffhersteller und der zunehmende Zugang in ländlichen und halbstädtischen Gebieten fördern die Akzeptanz.
Markteinblick in Japan für Dengue-Impfstoffe
Der japanische Dengue-Impfstoffmarkt gewinnt an Dynamik und wird im Prognosezeitraum voraussichtlich eine beachtliche jährliche Wachstumsrate aufweisen. Die Nachfrage wird durch zunehmende Reisen in endemische Länder und gemeldete Dengue-Fälle bei heimkehrenden Reisenden angetrieben. Japans präziser Gesundheitsansatz sowie öffentliche und private Investitionen in die Prävention tropischer Krankheiten unterstützen die zunehmende Akzeptanz. Die Bemühungen der Regierung, Impfstoffe für zukünftige Ausbrüche zu lagern, und leistungsstarke Diagnosemöglichkeiten tragen ebenfalls zum Marktwachstum bei.
Markteinblick in China für Dengue-Impfstoffe
Der chinesische Dengue-Impfstoffmarkt hatte 2024 mit 44,9 % den größten Marktanteil im asiatisch-pazifischen Raum. Dies ist auf die steigende Zahl der Dengue-Fälle in den südlichen Provinzen, den Ausbau öffentlicher Impfprogramme und eine robuste Inlandsproduktion zurückzuführen. Der Fokus des Landes auf die Bekämpfung tropischer Krankheiten, gepaart mit hohen Investitionen in die Biotechnologieproduktion und die Impfstoffforschung und -entwicklung, treibt das rasante Wachstum voran. Der staatliche Schwerpunkt auf Gesundheitssicherheit und Notfallvorsorge gewährleistet die kontinuierliche Integration von Dengue-Impfstoffen in die breiteren Strukturen des öffentlichen Gesundheitswesens.
Marktanteil des Dengue-Impfstoffs
Die Dengue-Impfstoffindustrie wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (Frankreich)
- Novartis AG (Schweiz)
- GSK plc (Großbritannien)
- F. Hoffmann-La Roche Ltd. (Schweiz)
- Takeda Pharmaceutical Company Limited (Japan)
- BIO-MED (Indien)
- Intercept Pharmaceuticals, Inc. (Großbritannien)
- Emcure Pharmaceuticals Ltd (Indien)
- Changchun BCHT Biotechnology Co. (China)
- Novo Medi Sciences Pvt. Ltd. (Indien)
Neueste Entwicklungen auf dem globalen Dengue-Impfstoffmarkt
- Im Februar 2024 gaben Takeda und Biological E eine Zusammenarbeit zur Produktion von 50 Millionen Dengue-Impfstoffen bekannt, um die Bemühungen zur Bekämpfung der Krankheit zu verstärken. Darüber hinaus eröffnete Miltenyi Biotec das Hyderabad Center for Cell and Gene Therapy Services, das sich auf fortschrittliche therapeutische Lösungen konzentriert. Die BioAsia 2024 präsentierte bedeutende Durchbrüche in der Pharmaindustrie und hob Innovationen und Fortschritte im Gesundheitswesen hervor.
- Im April 2023 genehmigten die argentinischen Gesundheitsbehörden die Verwendung eines japanischen Dengue-Impfstoffs, der in zwei Dosen im Abstand von drei Monaten verabreicht werden muss. Diese Entscheidung stellt einen wichtigen Schritt zur Stärkung der Dengue-Prävention im Land dar. Das Zwei-Dosen-Schema soll eine optimale Immunität gegen die Krankheit gewährleisten.
- Im Mai 2023 gab Takeda bekannt, dass sein Dengue-Impfstoff QDENGA mehrere Zulassungen erhalten hat, darunter eine von der brasilianischen Gesundheitsbehörde im März 2023. Diese Zulassung erlaubt die Anwendung des Impfstoffs bei Personen im Alter von 4 bis 60 Jahren und bietet Schutz gegen alle vier Dengue-Virus-Serotypen. Takedas Fortschritte bei der Erlangung dieser Zulassungen unterstreichen die anhaltenden Bemühungen des Unternehmens, Dengue-Fieber wirksam zu bekämpfen.
- Im März 2021 gab Takeda Pharmaceutical Company Limited bekannt, dass sein Dengue-Impfstoffkandidat TAK-003 die Zulassung der Europäischen Arzneimittelagentur (EMA) zur Prävention von Krankheitsausbrüchen bei Personen im Alter von 4 bis 60 Jahren erhalten hat. Das Unternehmen plant, im Laufe des Jahres 2021 die behördliche Zulassung für diesen Impfstoff in mehreren Ländern zu beantragen, darunter Argentinien, Brasilien, Kolumbien, Indonesien, Malaysia, Mexiko, Singapur, Sri Lanka und Thailand. Dieser Schritt unterstreicht Takedas Engagement, den Zugang zur Dengue-Prävention weltweit zu erweitern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.