Globaler Markt für Herzsicherheitsdienste, nach Diensten (EKG-/Holter-Messungen, Blutdruckmessungen , In-vitro-Dienste zur Beurteilung der Herzsicherheit, Herz-Kreislauf-Bildgebung, Telemetrieüberwachung in Echtzeit, zentrales Überlesen von EKGs, nichtinvasive Herzbildgebung, physiologische Stresstests, gründliche QT-Studien, TQT- und Expositionsreaktionsmodellierung, Thrombozytenaggregation und andere Dienste), Phase (Phase 1, Phase 2 und Phase 3), Typ (Integrierte Dienste und eigenständige Dienste), Endbenutzer ( Pharma- und Biopharmaunternehmen, Auftragsforschungsinstitute und akademische und Forschungsinstitute), Branchentrends und Prognose bis 2029.
Marktanalyse und Einblicke
Der globale Markt für Herzsicherheitsdienste wird von Faktoren wie der zunehmenden Entwicklung neuer Medikamente, einer wachsenden Zahl aufstrebender Akteure und technologischer Innovationen angetrieben, die die Nachfrage steigern, sowie von steigenden Investitionen in Forschung und Entwicklung, die zum Marktwachstum führen. Derzeit finden verschiedene Forschungsstudien statt, die den Herstellern einen Wettbewerbsvorteil bei der Entwicklung neuer und innovativer Herzsicherheitssysteme verschaffen sollen, was voraussichtlich verschiedene andere Möglichkeiten auf dem Markt für Herzsicherheitsdienste bietet. Die strengen staatlichen Zulassungsvorschriften dürften das Wachstum jedoch behindern.

Der globale Marktbericht zu Herzsicherheitsdiensten enthält Einzelheiten zu Marktanteilen, neuen Entwicklungen und Produktpipeline-Analysen, den Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neu entstehende Umsatzbereiche, Änderungen der Marktvorschriften, Produktzulassungen, strategische Entscheidungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um die Analyse und das Marktszenario zu verstehen, kontaktieren Sie uns für ein Analystenbriefing. Unser Team hilft Ihnen dabei, eine Umsatzauswirkungslösung zu entwickeln, mit der Sie Ihr gewünschtes Ziel erreichen. Die Skalierbarkeit und Geschäftsausweitung der Einzelhandelseinheiten in den Entwicklungsländern verschiedener Regionen sowie die Partnerschaft mit Lieferanten für den sicheren Vertrieb von Maschinen- und Arzneimittelprodukten sind die Haupttreiber, die die Marktnachfrage im Prognosezeitraum angetrieben haben.
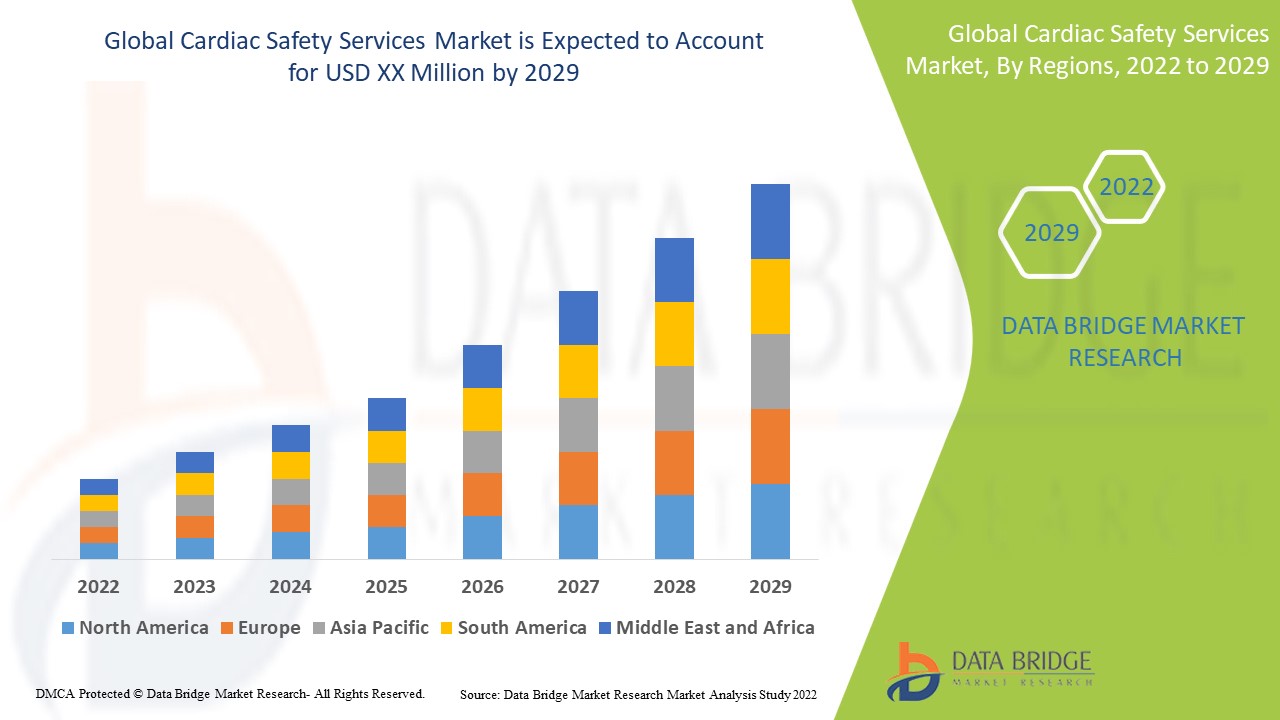
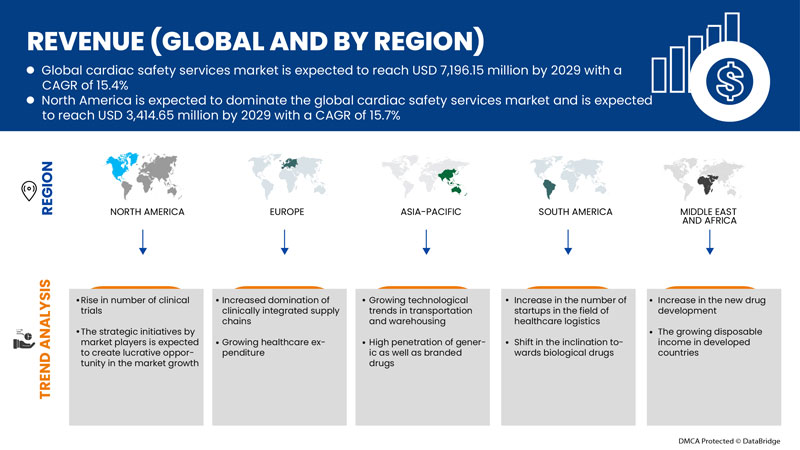
Der globale Markt für Herzsicherheitsdienste ist unterstützend und zielt darauf ab, das Fortschreiten der Krankheit zu verlangsamen. Data Bridge Market Research analysiert, dass der globale Markt für Herzsicherheitsdienste im Prognosezeitraum von 2022 bis 2029 mit einer durchschnittlichen jährlichen Wachstumsrate von 15,4 % wachsen wird.
|
Berichtsmetrik |
Details |
|
Prognosezeitraum |
2022 bis 2029 |
|
Basisjahr |
2021 |
|
Historische Jahre |
2020 (Anpassbar auf 2019 – 2014) |
|
Quantitative Einheiten |
Umsatz in Millionen USD, Preise in USD |
|
Abgedeckte Segmente |
Nach Services (EKG/Holter-Messungen, Blutdruckmessungen, In-vitro-Dienstleistungen zur Beurteilung der kardialen Sicherheit, kardiovaskuläre Bildgebung, Echtzeit-Telemetrieüberwachung, zentrales Überlesen von EKGs, nichtinvasive kardiale Bildgebung, physiologische Stresstests, gründliche QT-Studien, TQT- und Expositionsreaktionsmodellierung, Thrombozytenaggregation und andere Services), Phase (Phase 1, Phase 2 und Phase 3), Typ (Integrierte Services und eigenständige Services), Endnutzer (Pharma- und Biopharmaunternehmen, Vertragsforschungsinstitute und akademische und Forschungsinstitute) |
|
Abgedeckte Länder |
USA, Kanada, Mexiko, Brasilien, Argentinien und Rest von Südamerika, Südafrika, Saudi-Arabien, Vereinigte Arabische Emirate, Israel, Ägypten und Rest des Nahen Ostens und Afrikas, Deutschland, Frankreich, Großbritannien, Italien, Spanien, Türkei, Russland, Niederlande, Schweiz, Belgien und Rest von Europa, China, Japan, Südkorea, Indien, Australien, Singapur, Thailand, Malaysia, Indonesien, Philippinen und Rest des asiatisch-pazifischen Raums |
|
Abgedeckte Marktteilnehmer |
Koninklijke Philips NV, Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook, Celerion, Biotrial, NEXEL Co., Ltd, Richmond Pharmacology, PhysioStim, Shanghai Medicilon Inc, Clario, PPD Inc und andere. |
Marktdefinition
Herzsicherheitsdienste unterstützen und entwerfen im Allgemeinen klinische Studien und andere Forschungsarbeiten, die zur Überwachung der Herzsicherheit erforderlich sind. Die Nachfrage nach Herzsicherheitsdiensten ist sowohl in Industrie- als auch in Entwicklungsländern gestiegen. Der Grund dafür ist die zunehmende Anzahl klinischer Studien und Produkteinführungen. Der Markt für Herzsicherheitsdienste wächst aufgrund der Einführung innovativer Produkte, der Zunahme technologischer Produkte und des steigenden verfügbaren Einkommens. Der Markt wird im Prognosezeitraum aufgrund der Erschließung neuer Märkte, strategischer Initiativen von Marktteilnehmern und steigender Gesundheitsausgaben wachsen.
Globale Marktdynamik für Herzsicherheitsdienste
Treiber
- Zunahme der Anzahl klinischer Studien
Eine klinische Studie ist ein gut strukturiertes System, das Hunderte von Jahren alt ist und noch immer das Rückgrat der regulatorischen Anforderungen für die Zulassung eines Arzneimittels bildet. In letzter Zeit gab es im Bereich der klinischen Studien große Fortschritte, die die Anzahl klinischer Studien erhöht haben und das Marktwachstum ankurbeln dürften.
Es gab verschiedene Änderungen in der Regulierung der klinischen Studien, was die Anzahl der klinischen Studien und ihrer positiven Ergebnisse erhöht hat.
Zum Beispiel,
- Laut dem Artikel von Medical News ist die Zahl der Studien aufgrund der Verbesserung der Qualität klinischer Studien, wie z. B. der obligatorischen Schulung aller Mitarbeiter, deutlich gestiegen. Außerdem erklärte das NIH im Jahr 2017, dass alle Forscher und Mitarbeiter in Studien, die vom NIH finanziert werden, in guter klinischer Praxis (GCP) geschult werden sollten.
Anstieg der Gesundheitsausgaben und -finanzierung
Die Höhe der Mittel, die ein Land für sein Gesundheitswesen ausgibt, und ihre Wachstumsrate im Laufe der Zeit werden von einer Vielzahl wirtschaftlicher und sozialer Faktoren beeinflusst, einschließlich der Finanzierungsvereinbarungen und der Organisationsstruktur des Gesundheitssystems.
Die Gesundheitsausgaben sind in Industrie- und Schwellenländern gestiegen, da das verfügbare Einkommen der Bevölkerung wächst. Je mehr Geld für die Gesundheitsversorgung ausgegeben wird, desto gesünder ist die Bevölkerung eines Landes.

Gelegenheit
- Zunahme der Entwicklung neuer Medikamente
Die klinischen Studien sind für die Entdeckung und Entwicklung neuer Medikamente zur Behandlung von Krankheiten von entscheidender Bedeutung. Sie sind für Forscher die beste Möglichkeit, herauszufinden, welche Behandlungen bei Menschen wirken und welche nicht. Die Arzneimittelentwicklung ist gekennzeichnet durch die Entwicklung neuer Behandlungsmethoden wie Medikamente oder Geräte zur Heilung verschiedener Krankheiten wie Krebs, Hormonstörungen, Stoffwechselerkrankungen und anderer.
- Daher sind klinische Studien der effektivste Weg, um die Sicherheit und Wirksamkeit des Therapeutikums vor der Markteinführung und dem menschlichen Verzehr sicherzustellen. Dazu gehört auch die Bewertung der Herzsicherheit, die ein wesentlicher Bestandteil ist, bevor ein medizinisches Produkt auf den Markt kommt.
Einschränkung/Herausforderung
Die ordnungsgemäße Bewertung und Berichterstattung klinischer Daten zur Herzsicherheit ist von entscheidender Bedeutung. Zulassung und Produktrückruf für alle medizinischen Produkte hängen von der Bewertung der Herzsicherheit ab. Daher ist es notwendig, die Herzsicherheitsbewertung gemäß dem gesetzlichen Verfahren bereitzustellen und durchzuführen, da dies sonst zu einer verspäteten Zulassung des Produkts führt, was voraussichtlich das Marktwachstum bremsen wird.
Zum Beispiel,
- Dem Artikel von IQVIA zufolge gab es zwischen 1957 und 2007 47 Fälle von Rücknahmen von Medikamenten nach der Markteinführung, von denen 45 % auf Bedenken hinsichtlich der kardiovaskulären Toxizität zurückzuführen waren. Ebenso scheiterten in den letzten zwei Jahrzehnten 27 % der potenziellen neuen Arzneimittelmoleküle in der präklinischen Phase aufgrund von kardiovaskulärer Toxizität, da sie die erforderlichen regulatorischen Anforderungen nicht erfüllten.
Globale Marktsegmentierung für Herzsicherheitsdienste
Der globale Markt für Herzsicherheitsdienste wird nach Typ, Diensten, Phase und Endbenutzer kategorisiert. Das Wachstum zwischen den Segmenten hilft Ihnen bei der Analyse von Wachstumsnischen und Strategien zur Marktbearbeitung und zur Bestimmung Ihrer Hauptanwendungsbereiche und der Unterschiede in Ihren Zielmärkten.
Dienstleistungen
- EKG /Holter-Messungen
- Blutdruckmessungen
- In-vitro-Dienstleistungen zur Beurteilung der kardialen Sicherheit
- Kardiovaskuläre Bildgebung
- Telemetrieüberwachung in Echtzeit
- Zentrale Überablesung des EKGs
- Nicht-invasive Herzbildgebung
- Physiologische Stresstests
- Gründliche QT-Studien
- TQT und Expositionsreaktionsmodellierung
- Thrombozytenaggregation
- Weitere Dienstleistungen
Auf der Grundlage von Dienstleistungen ist der Markt für Herzsicherheitsdienste in EKG-/Holter-Messungen, Blutdruckmessungen, In-vitro-Herzsicherheitsbewertungsdienste, kardiovaskuläre Bildgebung, Echtzeit-Telemetrieüberwachung, zentrales Überlesen von EKGs, nicht-invasive Herzbildgebung, physiologische Stresstests, gründliche QT-Studien, TQT- und Expositionsreaktionsmodellierung, Thrombozytenaggregation und andere Dienste unterteilt.
Phase
- Phase 1
- Phase 2
- Phase 3
Auf der Grundlage der Phasen ist der Markt für Herzsicherheitsdienste in Phase 1, Phase 2 und Phase 3 unterteilt.
Typ
- Integrierte Dienste
- Standalone-Dienste
Auf der Grundlage des Typs ist der Markt für Herzsicherheitsdienste in integrierte Dienste und eigenständige Dienste segmentiert.
Endbenutzer
- Pharma- und Biopharmaunternehmen
- Auftragsforschungsinstitute
- Akademisches und Forschungsinstitut
Auf der Grundlage des Endbenutzers ist der Markt für Herzsicherheitsdienste in Pharma- und Biopharmaunternehmen, Auftragsforschungsinstitute sowie Hochschulen und Forschungsinstitute segmentiert.
Regionale Analyse/Einblicke zu Cardiac Safety Services
Die Herzsicherheitsdienste werden analysiert und es werden Einblicke in die Marktgröße und Trends nach Typ, Diensten, Phase und Endbenutzer wie oben angegeben bereitgestellt.
Die im Bericht zu kardiologischen Sicherheitsdiensten abgedeckten Länder sind die USA, Kanada, Mexiko, Brasilien, Argentinien und der Rest von Südamerika, Südafrika, Saudi-Arabien, die Vereinigten Arabischen Emirate, Israel, Ägypten und der Rest des Nahen Ostens und Afrikas, Deutschland, Frankreich, Großbritannien, Italien, Spanien, die Türkei, Russland, die Niederlande, die Schweiz, Belgien und der Rest von Europa, China, Japan, Südkorea, Indien, Australien, Singapur, Thailand, Malaysia, Indonesien, die Philippinen und der Rest des asiatisch-pazifischen Raums.
Aufgrund des technologischen Fortschritts in den Entwicklungsregionen wird erwartet, dass die USA dominieren werden.
Der Länderabschnitt des Berichts enthält auch einzelne marktbeeinflussende Faktoren und Änderungen der Marktregulierung, die die aktuellen und zukünftigen Trends des Marktes beeinflussen. Datenpunkte wie Downstream- und Upstream-Wertschöpfungskettenanalysen, technische Trends und Porters Fünf-Kräfte-Analyse sowie Fallstudien sind einige der Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Bereitstellung von Prognoseanalysen der Länderdaten werden auch die Präsenz und Verfügbarkeit globaler Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen inländischer Zölle und Handelsrouten berücksichtigt.
Wettbewerbsumfeld und Analyse der Herzsicherheitsdienste
Die Wettbewerbslandschaft des globalen Marktes für Herzsicherheitsdienste liefert Einzelheiten zu den Wettbewerbern. Zu den enthaltenen Details gehören Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen auf den Markt für Herzsicherheitsdienste.
Zu den wichtigsten Akteuren auf dem Markt zählen unter anderem Koninklijke Philips NV, Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook, Celerion, Biotrial, NEXEL Co., Ltd, Richmond Pharmacology, PhysioStim, Shanghai Medicilon Inc, Clario und PPD Inc.
Forschungsmethodik
Die Datenerfassung und die Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Die wichtigste Forschungsmethode, die das DBMR-Forschungsteam verwendet, ist die Datentriangulation, die Data Mining, Analyse der Auswirkungen von Datenvariablen auf den Markt und primäre (Branchenexperten-)Validierung umfasst. Abgesehen davon umfassen die Datenmodelle ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, einen Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Firmenmarktanteilsanalyse, Messstandards, eine globale vs. regionale und Lieferantenanteilsanalyse. Bitte fordern Sie bei weiteren Fragen einen Analystenanruf an.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CARDIAC SAFETY SERVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTERS FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6 INDUSTRY INSIGHT
6.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.2 PATENT ANALYSIS
6.3 PATENT FLOW DIAGRAM
6.4 KEY PATIENT ENROLLMENT STRATEGIES
6.5 PRICING STRATEGY
7 GLOBAL CARDIAC SAFETY SERVICES MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASE IN THE NUMBER OF CLINICAL TRIALS
8.1.2 INCREASE IN HEALTHCARE EXPENDITURE AND FUNDING
8.1.3 INCREASE IN STRATEGIC INITIATIVES BY MAJOR MARKET PLAYERS
8.1.4 INCREASE IN R&D ACTIVITIES
8.2 RESTRAINTS
8.2.1 HIGH COST OF CARDIAC SAFETY EVALUATION
8.2.2 STRICT REGULATORY
8.3 OPPORTUNITIES:
8.3.1 INCREASE IN NEW DRUG DEVELOPMENT
8.3.2 RISE IN THE EXPANSION OF THE CARDIAC SAFETY SERVICES
8.4 CHALLENGES
8.4.1 TIME-CONSUMING PROCEDURE
8.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CARDIAC SAFETY EVALUATION
9 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY SERVICES
9.1 OVERVIEW
9.2 ECG/HOLTER MEASUREMENTS
9.3 BLOOD PRESSURE MEASUREMENTS
9.4 IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES
9.4.1 HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS
9.4.1.1 1 CONCENTRATIONS
9.4.1.2 4 CONCENTRATIONS
9.4.2 COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA)
9.4.2.1 3 CONCENTRATIONS
9.4.2.2 5 CONCENTRATIONS
9.4.3 IN VITRO HERG ASSAY
9.4.4 OTHERS
9.5 CARDIOVASCULAR IMAGING
9.6 REAL TIME TELEMETRY MONITORING
9.7 CENTRAL OVER-READ OF ECGS
9.8 NON-INVASIVE CARDIAC IMAGING
9.9 PHYSIOLOGIC STRESS TESTING
9.1 THOROUGH QT STUDIES
9.11 TQT AND EXPOSURE RESPONSE MODELLING
9.12 PLATELET AGGREGATION
9.13 OTHERS
10 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY PHASE
10.1 OVERVIEW
10.2 PHASE I
10.3 PHASE II
10.4 PHASE III
11 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY TYPE
11.1 OVERVIEW
11.2 INTEGRATED SERVICES
11.3 STANDALONE SERVICES
12 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY END USER
12.1 OVERVIEW
12.2 PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES
12.3 CONTRACT RESEARCH ORGANIZATIONS
12.4 ACADEMIC AND RESEARCH INSTITUTE
13 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY REGION
13.1 OVERVIEW
13.2 NORTH AMERICA
13.2.1 U.S.
13.2.2 CANADA
13.2.3 MEXICO
13.3 EUROPE
13.3.1 GERMANY
13.3.2 FRANCE
13.3.3 U.K.
13.3.4 ITALY
13.3.5 SPAIN
13.3.6 TURKEY
13.3.7 RUSSIA
13.3.8 NETHERLANDS
13.3.9 SWITZERLAND
13.3.10 BELGIUM
13.3.11 REST OF EUROPE
13.4 ASIA-PACIFIC
13.4.1 CHINA
13.4.2 JAPAN
13.4.3 SOUTH KOREA
13.4.4 INDIA
13.4.5 AUSTRALIA
13.4.6 SINGAPORE
13.4.7 THAILAND
13.4.8 MALAYSIA
13.4.9 INDONESIA
13.4.10 PHILIPPINES
13.4.11 REST OF ASIA-PACIFIC
13.5 SOUTH AMERICA
13.5.1 BRAZIL
13.5.2 ARGENTINA
13.5.3 REST OF SOUTH AMERICA
13.6 MIDDLE EAST AND AFRICA
13.6.1 SOUTH AFRICA
13.6.2 SAUDI ARABIA
13.6.3 U.A.E.
13.6.4 ISRAEL
13.6.5 EGYPT
13.6.6 REST OF MIDDLE EAST AND AFRICA
14 GLOBAL CARDIAC SAFETY SERVICES MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 COMPANY PROFILE
15.1 EUROFINS SCIENTIFIC
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.1.5.1 AGREEMENTS
15.1.5.2 ACQUISITION
15.2 PPD INC. (SUBSIDIARY OF THERMO FISHER SCIENTIFIC INC)
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.2.5.1 INVESTMENT
15.3 KONINKLIJKE PHILIPS N.V.
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.3.5.1 ACQUISITION
15.4 IQVIA
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.4.5.1 ACQUISITION
15.5 LABORATORY CORPORATION OF AMERICA HOLDINGS
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.5.5.1 NEW LABORATORY
15.5.5.2 ACQUISITION
15.6 BANOOK
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.6.3.1 AGREEMENT
15.7 BIOTRIAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.7.3.1 NEW CENTER OPENING
15.8 CELERION
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.8.3.1 NEW CENTER OPENING
15.9 CERTARA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 CONTRACT
15.9.4.2 ACQUISITION
15.1 CLARIO
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.10.3.1 PRODUCT EXPANSION
15.11 MEDPACE
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.11.4.1 ACQUISITION
15.12 NCARDIA
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.12.3.1 PARTNERSHIP
15.13 NEXEL CO., LTD
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.13.3.1 JOINT VENTURE
15.13.3.2 PARTNERSHIP
15.14 PHYSIOSTIM
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.14.3.1 PARTNERSHIP
15.15 RICHMOND PHARMACOLOGY
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.15.3.1 EVENT
15.16 SGS SA
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.16.4.1 ACQUISITION
15.17 SHANGHAI MEDICILON INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENTS
15.17.3.1 PARTNERSHIP
15.17.3.2 PARTNERSHIP
16 QUESTIONNAIRE
17 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 2 PROBABILITY OF SUCCESS BY CLINICAL TRIAL PHASE TO THERAPEUTIC AREA
TABLE 3 MORTALITY RATES FROM CLINICAL TRIALS AND EUROPEAN SAFETY AND EXPOSURE SURVEY (ESES), DEATHS PER 100 (PYE)
TABLE 4 ADHERENCE RATE TO COMMON CARDIOVASCULAR MEDICATION
TABLE 5 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 6 INITIATIVES TO INCREASE ENROLLMENT IN CLINICAL TRIALS AMONG UNDERREPRESENTED POPULATIONS
TABLE 7 ESTIMATED COST OF CARDIAC SAFETY EVALUATION DEVICES
TABLE 8 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 9 GLOBAL ECG/HOLTER MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 GLOBAL BLOOD PRESSURE MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 GLOBAL IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 GLOBAL IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 13 GLOBAL HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 14 GLOBAL COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 15 GLOBAL CARDIOVASCULAR IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 GLOBAL REAL TIME TELEMETRY MONITORING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 GLOBAL CENTRAL OVER-READ OF ECGS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 GLOBAL NON-INVASIVE CARDIAC IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 GLOBAL PHYSIOLOGIC STRESS TESTING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 GLOBAL THOROUGH QT STUDIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 GLOBAL TQT AND EXPOSURE RESPONSE MODELLING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 GLOBAL PLATELET AGGREGATION IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 GLOBAL OTHERS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 25 GLOBAL PHASE I IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 GLOBAL PHASE II IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 GLOBAL PHASE III IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 29 GLOBAL INTEGRATED SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 GLOBAL STANDALONE SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 GLOBAL PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 GLOBAL CONTRACT RESEARCH ORGANIZATIONS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 GLOBAL ACADEMIC AND RESEARCH INSTITUTE IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 GLOBAL CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 U.S. CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 45 U.S. IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 46 U.S. HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 47 U.S. COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 48 U.S. CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 49 U.S. CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 50 U.S. CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 51 CANADA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 52 CANADA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 53 CANADA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 54 CANADA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 55 CANADA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 56 CANADA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 57 CANADA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 58 MEXICO CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 59 MEXICO IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 60 MEXICO HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 61 MEXICO COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 62 MEXICO CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 63 MEXICO CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 MEXICO CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 65 EUROPE CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 66 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 67 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 68 EUROPE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 69 EUROPE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 70 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 71 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 72 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 73 GERMANY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 74 GERMANY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 75 GERMANY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 76 GERMANY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 77 GERMANY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 78 GERMANY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 GERMANY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 80 FRANCE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 81 FRANCE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 82 FRANCE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 83 FRANCE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 84 FRANCE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 85 FRANCE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 86 FRANCE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 87 U.K. CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 88 U.K. IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 89 U.K. HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 90 U.K. COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 91 U.K. CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 92 U.K. CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 93 U.K. CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 ITALY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 95 ITALY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 96 ITALY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 97 ITALY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 98 ITALY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 99 ITALY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 100 ITALY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 101 SPAIN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 102 SPAIN IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 103 SPAIN HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 104 SPAIN COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 105 SPAIN CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 106 SPAIN CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 107 SPAIN CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 108 TURKEY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 109 TURKEY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 110 TURKEY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 111 TURKEY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 112 TURKEY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 113 TURKEY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 114 TURKEY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 115 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 116 RUSSIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 117 RUSSIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 118 RUSSIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 119 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 120 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 121 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 122 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 123 NETHERLANDS IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 124 NETHERLANDS HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 125 NETHERLANDS COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 126 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 127 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 128 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 129 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 130 SWITZERLAND IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 131 SWITZERLAND HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 132 SWITZERLAND COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 133 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 134 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 135 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 136 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 137 BELGIUM IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 138 BELGIUM HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 139 BELGIUM COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 140 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 141 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 143 REST OF EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 144 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 145 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 146 ASIA-PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 147 ASIA-PACIFIC HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 148 ASIA-PACIFIC COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 149 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 150 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 151 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 152 CHINA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 153 CHINA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 154 CHINA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 155 CHINA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 156 CHINA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 157 CHINA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 158 CHINA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 159 JAPAN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 160 JAPAN IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 161 JAPAN HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 162 JAPAN COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 163 JAPAN CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 164 JAPAN CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 165 JAPAN CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 166 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 167 SOUTH KOREA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 168 SOUTH KOREA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 169 SOUTH KOREA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 170 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 171 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 172 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 173 INDIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 174 INDIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 175 INDIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 176 INDIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 177 INDIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 178 INDIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 179 INDIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 180 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 181 AUSTRALIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 182 AUSTRALIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 183 AUSTRALIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 184 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 185 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 186 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 187 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 188 SINGAPORE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 189 SINGAPORE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 190 SINGAPORE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 191 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 192 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 193 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 194 THAILAND CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 195 THAILAND IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 196 THAILAND HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 197 THAILAND COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 198 THAILAND CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 199 THAILAND CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 200 THAILAND CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 201 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 202 MALAYSIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 203 MALAYSIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 204 MALAYSIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 205 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 206 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 207 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 208 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 209 INDONESIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 210 INDONESIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 211 INDONESIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 212 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 213 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 214 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 215 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 216 PHILIPPINES IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 217 PHILIPPINES HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 218 PHILIPPINES COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 219 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 220 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 221 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 222 REST OF ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 223 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 224 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 225 SOUTH AMERICA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 226 SOUTH AMERICA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 227 SOUTH AMERICA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 228 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 229 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 230 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 231 BRAZIL CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 232 BRAZIL IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 233 BRAZIL HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 234 BRAZIL COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 235 BRAZIL CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 236 BRAZIL CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 237 BRAZIL CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 238 ARGENTINA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 239 ARGENTINA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 240 ARGENTINA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 241 ARGENTINA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 242 ARGENTINA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 243 ARGENTINA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 244 ARGENTINA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 245 REST OF SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 246 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 247 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 248 MIDDLE EAST AND AFRICA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 249 MIDDLE EAST AND AFRICA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 250 MIDDLE EAST AND AFRICA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 251 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 252 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 253 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 254 SOUTH AFRICA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 255 SOUTH AFRICA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 256 SOUTH AFRICA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 257 SOUTH AFRICA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 258 SOUTH AFRICA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 259 SOUTH AFRICA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 260 SOUTH AFRICA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 261 SAUDI ARABIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 262 SAUDI ARABIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 263 SAUDI ARABIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 264 SAUDI ARABIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 265 SAUDI ARABIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 266 SAUDI ARABIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 267 SAUDI ARABIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 268 U.A.E. CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 269 U.A.E. IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 270 U.A.E. HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 271 U.A.E. COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 272 U.A.E. CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 273 U.A.E. CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 274 U.A.E. CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 275 ISRAEL CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 276 ISRAEL IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 277 ISRAEL HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 278 ISRAEL COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 279 ISRAEL CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 280 ISRAEL CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 281 ISRAEL CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 282 EGYPT CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 283 EGYPT IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 284 EGYPT HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 285 EGYPT COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 286 EGYPT CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 287 EGYPT CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 288 EGYPT CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 289 REST OF MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
Abbildungsverzeichnis
FIGURE 1 GLOBAL CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 2 GLOBAL CARDIAC SAFETY SERVICES MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL CARDIAC SAFETY SERVICES MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL CARDIAC SAFETY SERVICES MARKET: GLOBAL VS COUNTRY MARKET ANALYSIS
FIGURE 5 GLOBAL CARDIAC SAFETY SERVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL CARDIAC SAFETY SERVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL CARDIAC SAFETY SERVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 GLOBAL CARDIAC SAFETY SERVICES MARKET: MARKET END USER GRID
FIGURE 9 GLOBAL CARDIAC SAFETY SERVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR CARDIAC SAFETY SERVICES ARE EXPECTED TO DRIVE THE GLOBAL CARDIAC SAFETY SERVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 ECG/HOLTER MEASUREMENTS SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL CARDIAC SAFETY SERVICES MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM FOR ANY RANDOM DRUG
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL CARDIAC SAFETY SERVICES MARKET
FIGURE 15 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2021
FIGURE 16 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 17 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 18 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 19 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2021
FIGURE 20 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 21 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 22 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY PHASE, LIFELINE CURVE
FIGURE 23 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2021
FIGURE 24 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 25 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 26 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 27 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY END USER, 2021
FIGURE 28 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 29 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 GLOBAL CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 32 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY REGION (2021)
FIGURE 33 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY REGION (2022 & 2029)
FIGURE 34 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY REGION (2021 & 2029)
FIGURE 35 GLOBAL CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 36 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 37 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 38 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 39 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 40 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 41 EUROPE CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 42 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 43 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 44 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 45 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 46 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 47 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 48 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 49 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 50 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 51 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 52 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 53 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 54 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 55 SOUTH AMERICA CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 56 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 57 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 58 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 59 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 60 MIDDLE EAST AND AFRICA CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 61 GLOBAL CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)
FIGURE 62 NORTH AMERICA CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)
FIGURE 63 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)
FIGURE 64 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.