Global Antinuclear Antibody Test Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
3.00 Billion
USD
8.14 Billion
2024
2032
USD
3.00 Billion
USD
8.14 Billion
2024
2032
| 2025 –2032 | |
| USD 3.00 Billion | |
| USD 8.14 Billion | |
|
|
|
|
Globale Marktsegmentierung für antinukleäre Antikörpertests nach Antikörpertyp (Extrahierbare nukleäre Antigene (ENA), Anti-DSDNA und Histone, Anti-DFS70-Antikörper, Anti-PM-SCL, Anti-Centromere-Antikörper, Anti-SP100 und andere), Produkt (Instrumente, Verbrauchsmaterialien und Reagenzien sowie Dienstleistungen), Technik (ELISA, Indirekte Immunfluoreszenz (IIF), Blotting-Test, Antigen-Microarray, Gel-basierte Techniken, Multiplex-Assay, Durchflusszytometrie, Passive Hämagglutination (PHA) und andere), Anwendung (Autoimmunerkrankungen und Infektionskrankheiten), Endbenutzer (Krankenhäuser, Labore, Diagnosezentren, Forschungsinstitute und andere), Vertriebskanal (Direktausschreibung, Einzelhandel, Drittanbieter und andere) – Branchentrends und Prognose bis 2032
Marktgröße für Anti-Atom-Antikörpertests
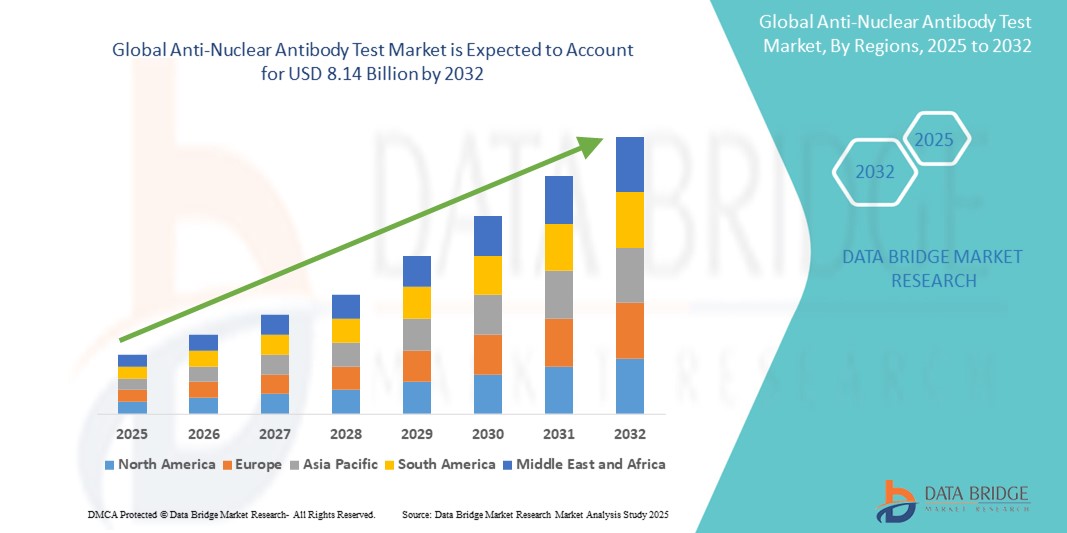
- Der globale Markt für Anti-Atom-Antikörpertests hatte im Jahr 2024 einen Wert von 3,00 Milliarden US-Dollar und dürfte bis 2032 einen Wert von 8,14 Milliarden US-Dollar erreichen , was einer jährlichen Wachstumsrate (CAGR) von 13,30 % im Prognosezeitraum entspricht.
- Dieses Wachstum ist auf Faktoren wie die zunehmende Verbreitung von Autoimmunerkrankungen wie systemischem Lupus erythematodes, rheumatoider Arthritis und Sjögren-Syndrom sowie die zunehmende Zahl älterer Menschen zurückzuführen.
Marktanalyse für Anti-Atom-Antikörpertests
- Der Anti-Atom-Antikörpertest ist eine wichtige diagnostische Methode zum Nachweis von Autoantikörpern, die Bestandteile des Zellkerns angreifen. Er unterstützt die Diagnose von Autoimmunerkrankungen wie systemischem Lupus erythematodes, rheumatoider Arthritis und systemischer Sklerose. Er ist bekannt für seine hohe Sensitivität, seinen klinischen Nutzen und die Fähigkeit, Krankheiten frühzeitig zu erkennen. Damit ist er ein unverzichtbares Instrument in der Diagnostik von Autoimmunerkrankungen.
- Der Markt für Anti-Atom-Antikörpertests verzeichnet ein stetiges Wachstum, das durch die steigende Zahl von Autoimmunerkrankungen, ein zunehmendes öffentliches Bewusstsein und Routine-Screening-Initiativen, kontinuierliche Fortschritte in der Diagnosetechnologie und den Ausbau der weltweiten Gesundheitsinfrastruktur vorangetrieben wird.
- Nordamerika wird voraussichtlich den Markt für Anti-Atom-Antikörpertests dominieren, da die Region über eine fortschrittliche Gesundheitsinfrastruktur verfügt und einen starken Schwerpunkt auf die Früherkennung von Krankheiten und personalisierte Medizin legt.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum aufgrund des zunehmenden Bewusstseins für Autoimmunerkrankungen und der steigenden Nachfrage nach frühzeitigen diagnostischen Eingriffen die am schnellsten wachsende Region im Markt für antinukleäre Antikörpertests sein.
- Das Segment der indirekten Immunfluoreszenz (IIF) wird voraussichtlich aufgrund seiner überlegenen Sensitivität und umfassenden diagnostischen Möglichkeiten den Markt dominieren. IIF bleibt die Goldstandardmethode für ANA-Tests, da sie ein breites Spektrum an Autoantikörpern erkennen kann, die mit verschiedenen Autoimmunerkrankungen assoziiert sind, darunter systemischer Lupus erythematodes (SLE) und das Sjögren-Syndrom. Die Fähigkeit, verschiedene Färbungsmuster zu erkennen, hilft Klinikern zudem, Ergebnisse besser zu interpretieren und zwischen bestimmten Autoimmunerkrankungen zu differenzieren, was für eine präzise Diagnose und Behandlungsplanung entscheidend ist.
Berichtsumfang und Marktsegmentierung für Anti-Atom-Antikörpertests
|
Eigenschaften |
Wichtige Markteinblicke zum Anti-Atom-Antikörpertest |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen. |
Markttrends für Anti-Atom-Antikörpertests
„Autoimmunerkrankungen stehen zunehmend im Fokus der Forschung“
- Ein wichtiger Trend auf dem globalen Markt für Anti-Atom-Antikörpertests sind die zunehmenden Forschungsanstrengungen im Bereich Autoimmunerkrankungen.
- Dieser Trend wird durch die weltweit steigende Belastung durch Autoimmunerkrankungen, das gestiegene wissenschaftliche Interesse am Verständnis der Krankheitsmechanismen und den Drang nach präziseren und frühzeitigeren Diagnoseinstrumenten vorangetrieben.
- So finanzieren und betreiben beispielsweise Organisationen wie die National Institutes of Health (NIH) und Pharmaunternehmen wie Roche und Eli Lilly aktiv Forschungen zur Identifizierung neuer Autoantikörper und zur Verbesserung der diagnostischen Genauigkeit bei Erkrankungen wie systemischem Lupus erythematodes und rheumatoider Arthritis.
- Der Schwerpunkt der Forschung zu Autoimmunerkrankungen nimmt in Industrieländern wie Nordamerika und Europa zu, ebenso wie in Schwellenländern, wo sich Investitionen im Gesundheitswesen und diagnostische Möglichkeiten rasch weiterentwickeln.
- Da die Wissenschaft immer tiefere Einblicke in die Autoimmunität gewinnt, wird die Nachfrage nach zuverlässigen und fortschrittlichen Anti-Atom-Antikörpertests voraussichtlich steigen. Dies wird Innovationen vorantreiben und die zukünftige Landschaft der Autoimmundiagnostik prägen.
Marktdynamik für Anti-Atom-Antikörpertests
Treiber
„Ausbau von Diagnosezentren und Laboren“
- Der weltweite Ausbau von Diagnosezentren und Laboren treibt das Wachstum des globalen Marktes für Anti-Atom-Antikörpertests maßgeblich voran. Mit der Weiterentwicklung der Gesundheitssysteme und der steigenden Nachfrage nach präziser und zeitnaher Diagnostik werden weitere Einrichtungen geschaffen, um diesem Bedarf gerecht zu werden.
- Diese Zentren spielen eine entscheidende Rolle bei der Verbesserung des Zugangs zu diagnostischen Tests und erleichtern den Patienten die Durchführung notwendiger Untersuchungen bei Autoimmunerkrankungen.
- Dank technologischer Fortschritte und verbesserter Laborkapazitäten können diese Einrichtungen nun ein breiteres Spektrum an Tests anbieten, darunter auch Tests auf antinukleäre Antikörper, die für die Diagnose verschiedener Autoimmunerkrankungen von entscheidender Bedeutung sind.
- Darüber hinaus ermöglicht die Verbreitung spezialisierter Labore schnellere Durchlaufzeiten und präzisere Ergebnisse, wodurch das Vertrauen der Ärzte in die Diagnose und Behandlungsplanung gestärkt wird.
- Dieser Wandel wird durch die zunehmende Fokussierung auf Früherkennung und präventive Gesundheitsfürsorge noch verstärkt, was die Bedeutung umfassender Tests unterstreicht.
Zum Beispiel,
- Laut einem Artikel von Living Media India Limited will Apollo Health & Lifestyle, eine Tochtergesellschaft von Apollo Hospitals Enterprise Limited, im April 2024 ihre Präsenz in Indien ausbauen und gleichzeitig ihre führende Position in der Diagnostik und Präventivmedizin behaupten. Der Ausbau der Diagnostikeinrichtungen wird den Zugang zu ANA-Tests deutlich verbessern und die Marktexpansion vorantreiben.
- Mit der Weiterentwicklung der Gesundheitssysteme steigt die Nachfrage nach präzisen und zugänglichen Diagnosedienstleistungen, was zur Einrichtung weiterer Einrichtungen führt. Dieser anhaltende Ausbau von Diagnosezentren und Laboren trägt maßgeblich zum Wachstum des Marktes für Anti-Atom-Antikörpertests bei.
Gelegenheit
„Integration digitaler Gesundheitslösungen“
- Die Integration digitaler Gesundheitslösungen bietet dem globalen Markt für Anti-Nuklear-Antikörper-Tests (ANA) zahlreiche Chancen und verändert den Zugang und die Nutzung von Tests. Einer der wichtigsten Vorteile ist der verbesserte Patientenzugang zu diagnostischen Leistungen.
- Telemedizin-Plattformen erfreuen sich zunehmender Beliebtheit. Sie ermöglichen es Patienten, medizinisches Fachpersonal aus der Ferne zu konsultieren und Empfehlungen für ANA-Tests zu erhalten, ohne dass geografische Entfernungen oder persönliche Besuche erforderlich sind.
- Diese Bequemlichkeit kann zu höheren Testraten führen, da Patienten eher Tests in Anspruch nehmen, wenn diese leicht zugänglich sind.
Zum Beispiel,
- Siemens Healthineers hat digitale Diagnostikplattformen eingeführt, die Labordaten, einschließlich Autoantikörper-Testergebnissen, in zentralisierte digitale Gesundheitsakten integrieren, um schnellere klinische Entscheidungen und bessere Patientenergebnisse zu ermöglichen.
- Da die Gesundheitssysteme die digitale Transformation weiter vorantreiben, dürfte die Einführung vernetzter Diagnosetechnologien neue Wachstumschancen für den Markt für Anti-Atom-Antikörpertests eröffnen.
Einschränkung/Herausforderung
„Mangelnde Standardisierung der Testprotokolle“
- Der Mangel an Standardisierung der Testprotokolle auf dem globalen Markt für Anti-Nuklear-Antikörper (ANA)-Tests stellt eine erhebliche Einschränkung dar, da er zu Inkonsistenzen bei der Durchführung und Interpretation der Tests in verschiedenen Laboren und Regionen führt.
- Unterschiedliche Methoden, Reagenzien und Richtlinien zur Ergebnisinterpretation führen zu Diskrepanzen in der Sensitivität und Spezifität von ANA-Tests, was es für Gesundheitsdienstleister schwierig macht, sich auf konsistente Ergebnisse zu verlassen
- Diese Inkonsistenz dürfte den klinischen Nutzen von ANA-Tests beeinträchtigen, da Ärzte aufgrund widersprüchlicher oder unklarer Ergebnisse möglicherweise Schwierigkeiten haben, genaue Diagnosen zu stellen. Ohne standardisierte Testprotokolle wird es für Hersteller zudem schwierig, allgemein anerkannte Testkits zu entwickeln, was die weltweite Akzeptanz einschränkt.
Zum Beispiel,
- Die Anwendung der indirekten Immunfluoreszenz (IIF) auf HEp-2-Zellen kann je nach Substratqualität, Verdünnungsgrenzwerten und Musterinterpretationskriterien zu unterschiedlichen Ergebnissen führen. Während einige Labore die Richtlinien des Internationalen Konsenses über ANA-Muster (ICAP) befolgen, tun dies andere nicht, was zu Abweichungen in der Testsensitivität und -spezifität führt.
- Das Fehlen standardisierter Testprotokolle auf dem globalen Markt für Anti-Nuklear-Antikörper (ANA)-Tests erschwert die Konsistenz der Testergebnisse und erschwert es medizinischen Fachkräften, dem Test zu vertrauen und ihn zuverlässig anzuwenden. Diese Inkonsistenz erschwert die Diagnose und verlangsamt die behördlichen Zulassungen sowie die weltweite Einführung. Dies schränkt die allgemeine Wirksamkeit des Tests in der klinischen Praxis ein.
Marktumfang für Anti-Atom-Antikörpertests
Der Markt ist nach Antikörpertyp, Produkt, Technik, Anwendung, Endbenutzer und Vertriebskanal segmentiert.
|
Segmentierung |
Untersegmentierung |
|
Nach Antikörpertyp |
|
|
Nach Produkt |
|
|
Nach Technik |
|
|
Nach Anwendung |
|
|
Nach Endbenutzer |
|
|
Nach Vertriebskanal |
|
Im Jahr 2025 wird die indirekte Immunfluoreszenz (IIF) voraussichtlich den Markt dominieren und den größten Anteil im Techniksegment haben.
Das Segment der indirekten Immunfluoreszenz (IIF) wird voraussichtlich im Jahr 2025 den Markt für Anti-Atom-Antikörpertests aufgrund seiner überlegenen Sensitivität und umfassenden diagnostischen Möglichkeiten dominieren. IIF bleibt die Goldstandardmethode für ANA-Tests, da sie ein breites Spektrum an Autoantikörpern erkennen kann, die mit verschiedenen Autoimmunerkrankungen assoziiert sind, darunter systemischer Lupus erythematodes (SLE) und das Sjögren-Syndrom. Die Fähigkeit, verschiedene Färbemuster zu erkennen, hilft Klinikern zudem, Ergebnisse besser zu interpretieren und zwischen bestimmten Autoimmunerkrankungen zu differenzieren, was für eine präzise Diagnose und Behandlungsplanung entscheidend ist.
Es wird erwartet, dass die rheumatoide Arthritis im Prognosezeitraum den größten Anteil im Anwendungssegment ausmachen wird
Im Jahr 2025 wird das Segment der rheumatoiden Arthritis voraussichtlich den Markt dominieren. Grund dafür ist die weltweit hohe Prävalenz der Erkrankung und die starke klinische Bedeutung von Tests auf antinukleäre Antikörper (ANA) für eine frühzeitige und genaue Diagnose. Rheumatoide Arthritis ist eine häufige Autoimmunerkrankung, die häufig eine regelmäßige Überwachung und Früherkennung erfordert, um Gelenkschäden vorzubeugen und die Behandlungsergebnisse zu verbessern. Die zunehmende Alterung der Bevölkerung, das gestiegene Bewusstsein für rheumatoide Arthritis und Fortschritte in der Diagnosetechnologie tragen zusätzlich zur führenden Position dieses Segments im ANA-Testmarkt bei.
Regionale Analyse des Marktes für Anti-Atom-Antikörpertests
„Nordamerika hält den größten Anteil am Markt für Anti-Atom-Antikörpertests“
- Nordamerika dominiert den Markt für Anti-Atom-Antikörpertests , was auf die fortschrittliche Gesundheitsinfrastruktur der Region und den starken Schwerpunkt auf die Früherkennung von Krankheiten und personalisierte Medizin zurückzuführen ist.
- Die USA haben einen erheblichen Anteil aufgrund der hohen Prävalenz von Autoimmunerkrankungen wie systemischem Lupus erythematodes und rheumatoider Arthritis, zusammen mit robusten Gesundheitsausgaben und einem weit verbreiteten Bewusstsein für diagnostische Tests
- Die regionale Führungsposition wird durch die Präsenz führender Diagnostikunternehmen wie Thermo Fisher Scientific und Bio-Rad Laboratories, starke regulatorische Rahmenbedingungen und kontinuierliche Investitionen in Forschung und Entwicklung im Bereich der Autoimmundiagnostik weiter unterstützt.
- Mit einem wachsenden Fokus auf Frühdiagnose, der Einführung fortschrittlicher Diagnoseplattformen und der Integration digitaler Gesundheitstechnologien wird Nordamerika voraussichtlich seine beherrschende Stellung auf dem globalen Markt für Anti-Atom-Antikörpertests bis 2032 behaupten.
„Im asiatisch-pazifischen Raum wird voraussichtlich die höchste jährliche Wachstumsrate im Markt für Anti-Atom-Antikörpertests verzeichnet“
- Im asiatisch-pazifischen Raum wird das höchste Wachstum im Markt für Anti-Atom-Antikörpertests erwartet , angetrieben durch das zunehmende Bewusstsein für Autoimmunerkrankungen und die steigende Nachfrage nach frühzeitigen Diagnosemaßnahmen.
- China hat aufgrund seiner schnell alternden Bevölkerung, der zunehmenden Verbreitung von Autoimmunerkrankungen und steigender Investitionen in die diagnostische Infrastruktur und den Zugang zur Gesundheitsversorgung einen erheblichen Anteil.
- Die Marktexpansion der Region wird auch durch steigende Gesundheitsausgaben, staatliche Initiativen zur Verbesserung des Krankheitsscreenings und eine wachsende Beteiligung des privaten Sektors an diagnostischen Dienstleistungen unterstützt.
- Dank verbessertem Zugang zu Gesundheitsdienstleistungen, wachsendem Versicherungsschutz und steigender Nachfrage nach fortschrittlichen Diagnoselösungen dürfte der asiatisch-pazifische Raum bis 2032 das globale Marktwachstum für Anti-Atom-Antikörpertests anführen.
Marktanteil von Anti-Atom-Antikörpertests
Die Wettbewerbslandschaft des Marktes liefert detaillierte Informationen zu den einzelnen Wettbewerbern. Zu den Details gehören Unternehmensübersicht, Unternehmensfinanzen, Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben genannten Datenpunkte beziehen sich ausschließlich auf die Marktausrichtung der Unternehmen.
Die wichtigsten Marktführer auf dem Markt sind:
- Abbott (USA)
- Abcam Limited (Großbritannien)
- Antibodies Incorporated (USA)
- Bio-Rad Laboratories, Inc. (USA)
- BioVision Inc. (USA)
- Grifols, SA (Spanien)
- Immuno Concepts Ltd. (USA)
- Merck KGaA (Deutschland)
- Orgentec Diagnostika GmbH (Deutschland)
- Revvity, Inc. (USA)
- Quidel Corporation (USA)
- Seramun Diagnostica GmbH (Deutschland)
- Thermo Fisher Scientific Inc. (USA)
- Transasia Bio-Medicals Ltd. (Indien)
- Trinity Biotech plc (Irland)
- Werfen SA (Spanien)
- ZEUS Scientific, Inc. (USA)
Neueste Entwicklungen auf dem globalen Markt für Anti-Atom-Antikörpertests
- Im September 2024 haben A. Menarini Diagnostics und Nucleix eine strategische Partnerschaft zur Einführung eines nicht-invasiven Blasenkrebstests in Europa geschlossen. Ziel dieser Zusammenarbeit ist es, die Früherkennung und Diagnose von Blasenkrebs zu verbessern und Patienten und Gesundheitsdienstleistern erhebliche Vorteile zu bieten. Für Menarini stärkt diese Partnerschaft das Portfolio und positioniert das Unternehmen als führenden Anbieter innovativer Diagnoselösungen.
- Im Mai 2023 haben Thermo Fisher und BRIN eine Partnerschaft geschlossen, um die Forschungskapazitäten in Indonesien zu verbessern. Der Schwerpunkt liegt dabei auf der Förderung wissenschaftlicher Innovationen und der Zusammenarbeit in den Bereichen Biowissenschaften, Biotechnologie und Umweltstudien für lokale Forscher.
- Im Januar 2023 brachte Revvitys EUROIMMUN ein automatisiertes indirektes Immunfluoreszenztestsystem (IIFT) auf den Markt, das die diagnostische Genauigkeit und Effizienz beim Nachweis von Autoantikörpern verbessert. Diese Innovation optimiert Laborabläufe, reduziert manuelle Fehler und ermöglicht es EUROIMMUN, die steigende Nachfrage zu bedienen. Dies steigert letztendlich das Umsatzwachstum und unterstreicht Revvitys Engagement für transformative Gesundheitslösungen.
- Im Januar 2023 gab Quantum-Si eine Zusammenarbeit mit Aviva Systems Biology bekannt, um Proteinanreicherungskits für eine verbesserte Proteinsequenzierung zu entwickeln. Die Kits enthalten Immunpräzipitationstools, um Arbeitsabläufe zu optimieren und eine detaillierte Analyse von Proteinvarianten zu ermöglichen. Dies erleichtert die Erforschung biologischer Prozesse und Krankheiten.
- Im November 2022 hat Bio-Rad sein Angebot an Qualitätskontrollen speziell für die klinischen Diagnostikplattformen von Abbott erweitert und so die Leistung und Zuverlässigkeit der Labore verbessert. Diese Initiative verbessert die diagnostische Genauigkeit und die Patientenversorgung und stärkt gleichzeitig die Wettbewerbsposition von Bio-Rad im Gesundheitsmarkt. Mit innovativen Qualitätskontrolllösungen will Bio-Rad den wachsenden Anforderungen der Labore gerecht werden, das Umsatzwachstum steigern und sein Engagement für die Weiterentwicklung diagnostischer Exzellenz unterstreichen.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.