Europe Endotoxin And Pyrogen Testing Market
Marktgröße in Milliarden USD
CAGR :
% 
 758.60
1,167.74
2020
2028
758.60
1,167.74
2020
2028
| 2021 –2028 | |
| USD 758.60 | |
| USD 1,167.74 | |
|
|
|
Europa Endotoxin und Pyrogen-Test-Markt, nach Produkttyp (Erkennung Kits & Reagenzien, Instrumente, Systeme und Software, Endotoxin-Test-Dienstleistungen und Verbrauchsmaterialien & Zubehör), Testtyp (Limulus Amöbozyten Lysat (LAL) Test, TAL-Test, Monozyten-Aktivierung Test (MAT), Kaninchen Pyrogen-Test und Rekombinant C (RFC) Assay), Anwendung (Pharmazeutische Herstellung, Medizinprodukte Herstellung, Rohstoffe Produktion und Verpackung Herstellung), Methode (Gel Clot Endotoxin Test, Chromogen Endotoxin Test und Turbidimetric Endotoxin Test), Art des Kaufs (Große Gruppe, mittlere und kleine Gruppe und Einzelperson), Endprodukt (Impfstoff und / oder CGT, Biologika , injizierbar und andere) Endbenutzer (Pharmazeutische Unternehmen, Biotechnologie-Unternehmen, Biomedizin-Unternehmen, Medizinprodukte-Unternehmen, Contract Research Organization (CRO) und Contract Manufacturing Organization (CMO), Land (Großbritannien, Deutschland, Frankreich, Spanien, Italien, Niederlande, Schweiz, Russland, Belgien und Rest von Europa) Industrie-Trends und Prognose zu 2028
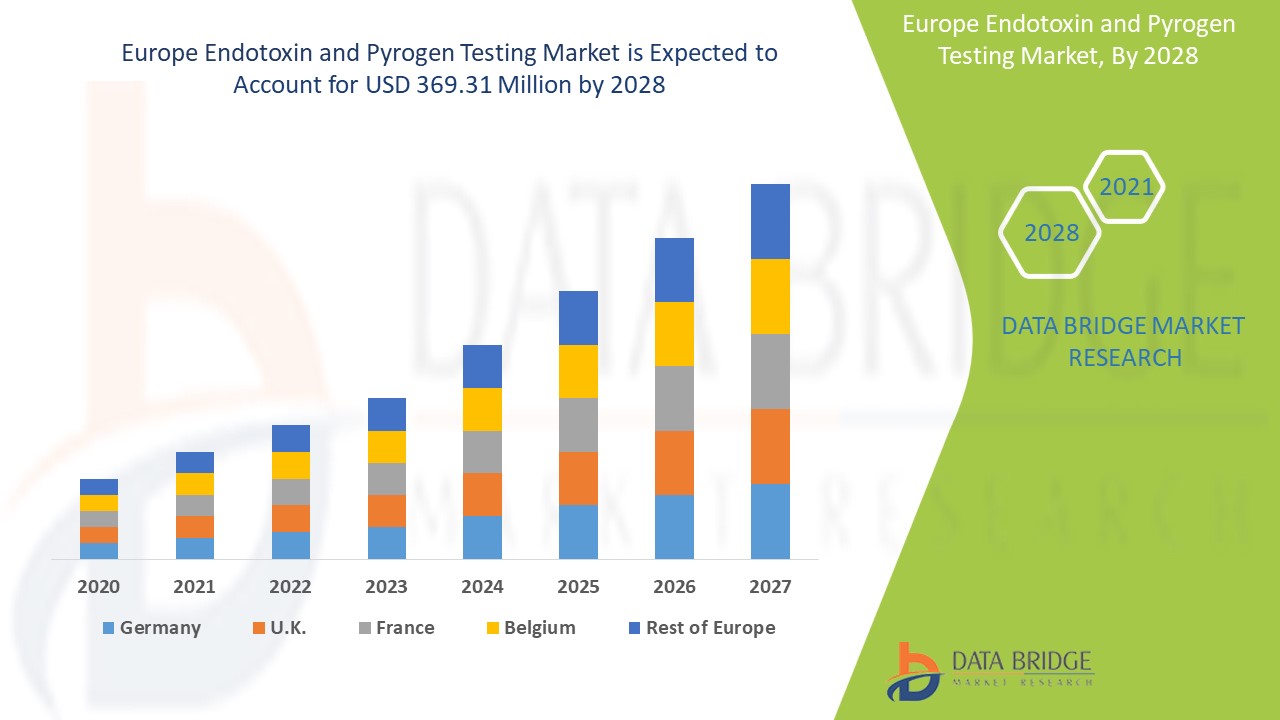 Marktanalyse und Einblicke: Europäischer Markt für Endotoxin- und Pyrogentests
Marktanalyse und Einblicke: Europäischer Markt für Endotoxin- und Pyrogentests
Der europäische Markt für Endotoxin- und Pyrogentests wird voraussichtlich von 173,90 Millionen US-Dollar im Jahr 2020 auf 369,31 Millionen US-Dollar im Jahr 2028 anwachsen und im Prognosezeitraum von 2021 bis 2028 eine CAGR von 10,5 % aufweisen. Die steigende Nachfrage nach Produkten für Endotoxintests in der Pharma- und Biotechnologiebranche treibt das Wachstum des Marktes voran.
Endotoxin ist eine Art Pyrogen und Bestandteil der äußeren Zellmasse von Gram-negativen Bakterien wie E. coli. Endotoxin ist ein Lipopolysaccharid oder LPS. LPS besteht aus dem Lipid, einem Teil, der ungesättigte Fette und Disaccharidphosphate enthält, Kernpolysacchariden und dem O-Antigen.
Die steigende Nachfrage nach Endotoxintests in der Pharma- und Biotechnologiebranche sowie der technologische Fortschritt bei der Entwicklung neuer Endotoxintestmethoden dürften das Wachstum des Marktes für Endotoxin- und Pyrogentests vorantreiben. Strategische Initiativen wichtiger Marktteilnehmer zur Markterweiterung wirken ebenfalls als Wachstumstreiber für den Markt für Endotoxin- und Pyrogentests. Die strengen staatlichen Vorschriften zur Verwendung von Tieren und die Variabilität des LAL-Tests wirken sich jedoch hemmend auf das Wachstum des Marktes für Endotoxin- und Pyrogentests aus.
- Im Oktober 2020 brachte Lonza das neue PyroCell MAT-System auf den Markt, das eine empfindliche und nachhaltige Erkennung einer breiten Palette von Pyrogenen ermöglicht, ohne dass Versuchstiere erforderlich sind. Das Produkt wurde als Komplettlösung mit Medien und Zubehör von Lonza und optimierten MAT-Reagenzien von Sanquin auf den Markt gebracht. Dieses neue Produkt des Unternehmens hat seine Glaubwürdigkeit und Nachfrage auf dem Markt erhöht.
Der Bericht zum Endotoxin- und Pyrogenmarkt enthält Einzelheiten zu Marktanteilen, neuen Entwicklungen und Produktpipeline-Analysen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neu entstehende Umsatzbereiche, Änderungen der Marktvorschriften, Produktzulassungen, strategische Entscheidungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um die Analyse und das Marktszenario zu verstehen, kontaktieren Sie uns für ein Analyst Briefing. Unser Team hilft Ihnen dabei, eine Umsatzauswirkungslösung zu entwickeln, mit der Sie Ihr gewünschtes Ziel erreichen.
 Europa Endotoxin- und Pyrogentests Marktumfang und Marktgröße
Europa Endotoxin- und Pyrogentests Marktumfang und Marktgröße
Der europäische Markt für Endotoxin- und Pyrogentests ist in sieben wichtige Segmente unterteilt: Produkttyp, Testtyp, Anwendung, Methode, Kaufart, Endprodukt und Endbenutzer. Das Wachstum zwischen den Segmenten hilft Ihnen bei der Analyse von Wachstumsnischen und Strategien zur Marktbearbeitung und zur Bestimmung Ihrer wichtigsten Anwendungsbereiche und der Unterschiede in Ihren Zielmärkten.
- Auf der Grundlage des Produkttyps ist der Markt für Endotoxin- und Pyrogentests in Nachweiskits und Reagenzien, Instrumente, Systeme und Software, Endotoxintestdienste sowie Verbrauchsmaterialien und Zubehör unterteilt. Im Jahr 2021 dominiert das Segment Nachweiskits und Reagenzien den Markt mit dem größten Marktanteil.
- Auf der Grundlage des Testtyps ist der Markt für Endotoxin- und Pyrogentests in Limulus-Amöbozytenlysat-Test (LAL), TAL-Test, Monozytenaktivierungstest (MAT), Kaninchen-Pyrogentest und rekombinanten C-Test (RFC) unterteilt. Im Jahr 2021 dominiert das Limulus-Amöbozytenlysat-Testsegment (LAL) den Markt mit dem größten Marktanteil.
- Auf der Grundlage der Anwendung ist der Markt für Endotoxin- und Pyrogentests in die Bereiche Arzneimittelherstellung, Herstellung medizinischer Geräte, Rohstoffproduktion und Verpackungsherstellung unterteilt. Im Jahr 2021 dominiert das Segment der Arzneimittelherstellung den Markt mit dem größten Marktanteil.
- Auf der Grundlage der Methode ist der Markt für Endotoxin- und Pyrogentests in Gel-Clot-Endotoxin-Test, chromogenen Endotoxin-Test und turbidimetrischen Endotoxin-Test unterteilt. Im Jahr 2021 dominiert das Segment der Gel-Clot-Endotoxin-Tests den Markt mit dem größten Marktanteil.
- Auf der Grundlage der Kaufart ist der Markt für Endotoxin- und Pyrogentests in große Gruppen, mittlere und kleine Gruppen sowie Einzelpersonen unterteilt. Im Jahr 2021 dominiert das Segment der großen Gruppen den Markt mit dem größten Marktanteil.
- Auf der Grundlage des Endprodukts ist der Markt für Endotoxin- und Pyrogentests in Impfstoffe und/oder CGT, Biologika, Injektionsmittel und andere unterteilt. Im Jahr 2021 dominiert das Impfstoff- und/oder CGT-Segment den Markt mit dem größten Marktanteil.
- Auf der Grundlage des Endverbrauchers ist der Markt für Endotoxin- und Pyrogentests in Pharmaunternehmen, Biotechnologieunternehmen, biomedizinische Unternehmen, Medizinprodukteunternehmen, Auftragsforschungsinstitute (CRO) und Auftragsfertigungsinstitute (CMO) unterteilt. Im Jahr 2021 dominiert das Segment der Pharmaunternehmen den Markt mit dem größten Marktanteil.
Endotoxin und Pyrogen Markt – Länderebene Analyse
Der Markt für Endotoxin- und Pyrogentests wird analysiert und Informationen zur Marktgröße werden nach Produkttyp, Testtyp, Anwendung, Methode, Kaufart, Endprodukt und Endbenutzer wie oben angegeben bereitgestellt. Die im Bericht zum Endotoxin- und Pyrogenmarkt abgedeckten Länder sind Großbritannien, Deutschland, Frankreich, Spanien, Italien, Niederlande, Schweiz, Russland, Belgien und der Rest Europas.
Für Europa wird im Prognosezeitraum von 2020 bis 2028 mit der vielversprechendsten Wachstumsrate gerechnet, da die zunehmende Nutzung des Endotoxin- und Pyrogenmarkts zu einer steigenden Nachfrage nach Endotoxintests in der Pharma- und Biotechnologiebranche führt.
Der Länderabschnitt des Berichts enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Markttrends auswirken. Datenpunkte wie Neuverkäufe, Ersatzverkäufe, demografische Daten des Landes, Regulierungsgesetze und Import-/Exportzölle sind einige der wichtigsten Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Prognoseanalyse der Länderdaten werden auch die Präsenz und Verfügbarkeit europäischer Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen der Vertriebskanäle berücksichtigt.
Neue Produkteinführungen durch Hersteller schaffen neue Möglichkeiten für Akteure auf dem Markt für Endotoxin- und Pyrogentests
Der Endotoxin- und Pyrogenmarkt bietet Ihnen außerdem eine detaillierte Marktanalyse für jedes Land, das in der ästhetischen Industrie wächst, mit Endotoxin- und Pyrogenmarktverkäufen, Auswirkungen des Fortschritts auf den Endotoxin- und Pyrogenmarkt und Änderungen der regulatorischen Szenarien mit ihrer Unterstützung für den Endotoxin- und Pyrogenmarkt. Die Daten sind für den historischen Zeitraum 2010 bis 2019 verfügbar.
Wettbewerbsumfeld und Endotoxin und Pyrogen Marktanteilsanalyse
Die Wettbewerbslandschaft des Endotoxin- und Pyrogenmarkts liefert Details nach Wettbewerbern. Die enthaltenen Details sind Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, Produktionsstandorte und -anlagen, Stärken und Schwächen des Unternehmens, Produkteinführung, Produkttestpipelines, Produktzulassungen, Patente, Produktbreite und -breite, Anwendungsdominanz, Technologie-Lebenslinienkurve. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus des Unternehmens in Bezug auf den Endotoxin- und Pyrogenmarkt.
Zu den wichtigsten Akteuren auf dem Markt für Endotoxin- und Pyrogentests zählen unter anderem Eurofins Scientific, Thermo Fisher Scientific Inc., Pall Corporation (eine Tochtergesellschaft von Danaher), Lonza, Charles River Laboratories, Merck KGaA, STERIS plc, SGS SA, Sartorius AG, bioMérieux SA, Ellab A/S, Wako USA (eine Tochtergesellschaft von FUJIFILM Wako Pure Chemical Corporation), ASSOCIATES OF CAPE COD, INC., WuXi AppTec, Microcoat Biotechnologie GmbH, Sanquin, Reading Scientific Services Ltd, nanoComposix, Zwisler Laboratorium GmbH und GenScript. DBMR-Analysten kennen die Stärken der Konkurrenz und erstellen für jeden Wettbewerber eine separate Wettbewerbsanalyse.
Darüber hinaus werden von den Unternehmen weltweit zahlreiche Produkteinführungen und Vereinbarungen initiiert, die den Endotoxin- und Pyrogenmarkt ebenfalls ankurbeln.
Zum Beispiel,
- Im September 2019 brachte Charles River Laboratories neue Lösungen auf den Markt, die aktualisierte EndoScan-V-Softwareplattform und die automatisierte Celsis-Erkennungsplattform für Sterilität, um den Bedarf der Pharmaindustrie zu decken. Dieses neue Produkt des Unternehmens hat das Portfolio der mikrobiellen Lösungen des Unternehmens gestärkt und wird in Zukunft zu höheren Umsätzen und Erträgen führen.
- Im Juni 2019 führte Wako USA (eine hundertprozentige Tochtergesellschaft der japanischen FUJIFILM Wako Pure Chemical Corporation) die Produktreihe von Endotoxin-Testreagenzien mit dem Namen PYROSTAR ES-F-Reihe von Limulus-Amöbozytenlysat-(LAL)-Reagenzien ein. Diese neue Produkteinführung des Unternehmens könnte die Qualitätssicherung der in Zukunft entwickelten COVID-19-Impfstoffe unterstützen und zu einer erhöhten Nachfrage nach seinem Produkt auf dem Markt führen.
Zusammenarbeit, Joint Ventures und andere Strategien der Marktteilnehmer stärken den Unternehmensmarkt im Endotoxin- und Pyrogenmarkt, was den Unternehmen auch den Vorteil bietet, ihr Angebot für den Endotoxin- und Pyrogenmarkt zu verbessern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.













