Europe Digital Therapeutics Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
862.86 Million
USD
4,639.40 Million
2024
2032
USD
862.86 Million
USD
4,639.40 Million
2024
2032
| 2025 –2032 | |
| USD 862.86 Million | |
| USD 4,639.40 Million | |
|
|
|
|
Marktsegmentierung für digitale Therapeutika (DTx) in Europa nach Produkt (Lösungen/Software, Hardwareprodukte und Dienstleistungen), Anwendung (Behandlung, Prävention und andere), Endbenutzer (Krankenhäuser, Fachkliniken, häusliche Pflege und andere), Vertriebskanal (Direktausschreibung, Einzelhandel und andere) – Branchentrends und Prognose bis 2032
Marktgröße für digitale Therapeutika (DTx) in Europa
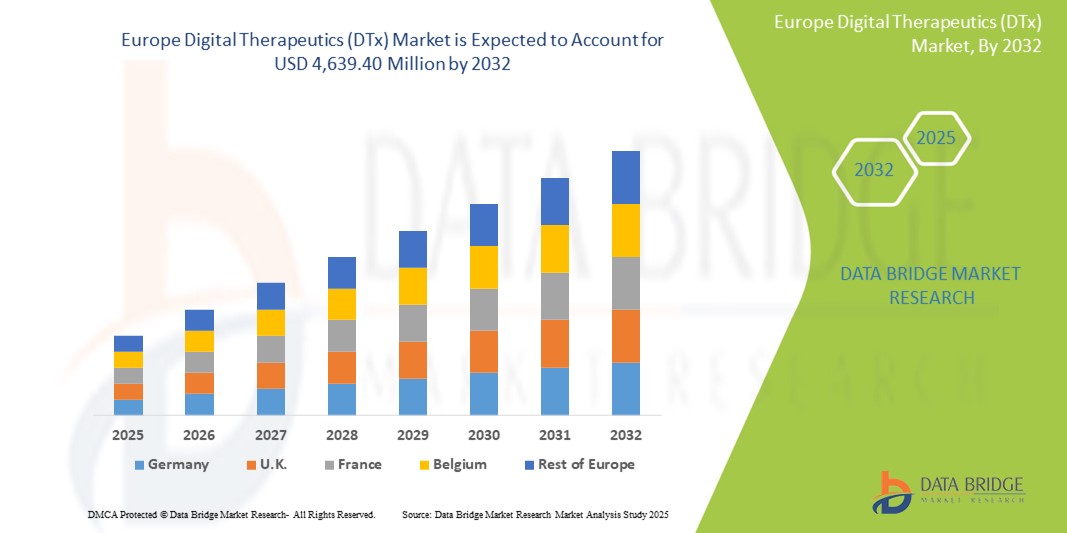
- Der europäische Markt für digitale Therapeutika (DTx) wurde im Jahr 2024 auf 862,86 Millionen US-Dollar geschätzt und soll bis 2032 4.639,40 Millionen US-Dollar erreichen , bei einer CAGR von 23,4 % im Prognosezeitraum.
- Das Marktwachstum wird vor allem durch die zunehmende Digitalisierung des Gesundheitswesens, die zunehmende Belastung durch chronische Krankheiten und die wachsende Nachfrage nach evidenzbasierten, personalisierten Therapielösungen in der gesamten Region vorangetrieben.
- Darüber hinaus stärken günstige regulatorische Rahmenbedingungen, verstärkte Investitionen in Innovationen im Gesundheitsbereich und die wachsende Akzeptanz digitaler Therapeutika bei medizinischem Fachpersonal und Patienten die Rolle von DTx als ergänzendes oder alternatives Therapieinstrument. Diese kombinierten Trends beschleunigen die Marktakzeptanz und treiben die nachhaltige Expansion des Sektors in ganz Europa voran.
Marktanalyse für digitale Therapeutika (DTx) in Europa
- Digitale Therapeutika, die evidenzbasierte therapeutische Interventionen über Software zur Vorbeugung, Behandlung oder Behandlung von Erkrankungen bereitstellen, werden in der sich entwickelnden Gesundheitslandschaft Europas aufgrund ihrer Skalierbarkeit, Kosteneffizienz und Fähigkeit, das Management chronischer Krankheiten und die psychische Gesundheit zu unterstützen, immer wichtiger.
- Die steigende Nachfrage nach DTx ist auf die zunehmende Verbreitung chronischer Krankheiten wie Diabetes , Herz-Kreislauf-Erkrankungen und psychischer Störungen zurückzuführen, gepaart mit der zunehmenden Akzeptanz digitaler Gesundheitstools sowohl bei Patienten als auch bei Gesundheitsdienstleistern.
- Deutschland dominierte den europäischen Markt für digitale Therapeutika (DTx) mit dem größten Umsatzanteil von 31,7 % im Jahr 2024. Dies ist auf die Initiative für digitale Gesundheitsanwendungen (DiGA) zurückzuführen, die die Erstattung verschriebener DTx ermöglicht, auf ein unterstützendes regulatorisches Umfeld und eine starke Integration digitaler Therapeutika in klinische Behandlungspfade.
- Spanien wird im Prognosezeitraum voraussichtlich das am schnellsten wachsende Land auf dem europäischen Markt für digitale Therapeutika (DTx) sein, unterstützt durch nationale Initiativen im Bereich der digitalen Gesundheit, die zunehmende Digitalisierung des Gesundheitswesens und die wachsende Nachfrage nach ferngesteuerten, patientenzentrierten Therapielösungen.
- Das Segment Lösungen/Software dominierte den europäischen Markt für digitale Therapeutika (DTx) mit einem Marktanteil von 65,2 % im Jahr 2024, was auf die einfache Bereitstellung, Skalierbarkeit und weit verbreitete Nutzung bei der Behandlung chronischer und psychischer Erkrankungen über mobile Apps und Cloud-basierte Plattformen zurückzuführen ist.
Berichtsumfang und Marktsegmentierung für digitale Therapeutika (DTx) in Europa
|
Eigenschaften |
Wichtige Markteinblicke für digitale Therapeutika (DTx) in Europa |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Europa
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für digitale Therapeutika (DTx) in Europa
„Umfassende Integration mit nationalen Gesundheitssystemen und Erstattungswegen“
- Ein wichtiger und sich beschleunigender Trend im europäischen DTx-Markt ist die Integration digitaler Therapeutika in öffentliche Gesundheitssysteme durch strukturierte Regulierungs- und Erstattungsrahmen. Länder wie Deutschland, Großbritannien und Frankreich sind Vorreiter bei diesem Übergang, indem sie die Verschreibung und Erstattung digitaler Gesundheitsinstrumente analog zu traditionellen Therapien formalisieren.
- So ermöglicht beispielsweise das deutsche Rahmenwerk für digitale Gesundheitsanwendungen (DiGA) die Verschreibung zertifizierter digitaler Therapeutika durch Ärzte und die Erstattung durch die gesetzlichen Krankenkassen. Dies fördert eine schnelle Einführung und Innovation. Auch Frankreich hat sein PECAN-Programm gestartet, das darauf abzielt, digitale Lösungen für die Behandlung chronischer Krankheiten in das öffentliche System zu integrieren.
- Dieser Wandel ermöglicht es DTx-Entwicklern, klinische Validierung und kommerzielle Nachhaltigkeit zu erreichen und gleichzeitig das Vertrauen von Ärzten und Patienten zu stärken. Die Rahmenbedingungen erfordern typischerweise strenge klinische Nachweise, die Einhaltung der Datensicherheit und die praktische Wirksamkeit, was den Markt zu höheren Qualitätsstandards drängt.
- Darüber hinaus steigert die Möglichkeit der Integration mit elektronischen Gesundheitsakten (EHRs) und nationalen digitalen Gesundheitsplattformen den Wertbeitrag der DTx-Tools weiter und bietet nahtlose Arbeitsabläufe für Ärzte und ganzheitliche Betreuungserlebnisse für Patienten.
- Da Regierungen in ganz Europa zunehmend das Potenzial von DTx zur Senkung der Gesundheitskosten und zur Verbesserung der Behandlung chronischer Krankheiten erkennen, wird erwartet, dass sich dieser Trend auf weitere Märkte und Therapiebereiche ausweitet.
- Die wachsende politische Unterstützung und institutionelle Förderung tragen nicht nur zur Verbreitung der Therapien bei, sondern verändern auch die Art und Weise, wie Therapien in den europäischen Gesundheitssystemen erbracht und erstattet werden.
Marktdynamik für digitale Therapeutika (DTx) in Europa
Treiber
„Steigende Belastung durch chronische Krankheiten und Nachfrage nach skalierbaren Pflegelösungen“
- Die zunehmende Verbreitung chronischer Krankheiten wie Diabetes, Bluthochdruck , Herz-Kreislauf-Erkrankungen und psychischer Störungen ist ein Haupttreiber für den Markt für digitale Therapeutika in Europa. Diese Erkrankungen erfordern eine langfristige, personalisierte Behandlung, für die traditionelle Gesundheitssysteme oft nicht in der Lage sind, diese in großem Umfang zu leisten.
- So kooperierte Sidekick Health im Januar 2024 mit Pfizer, um seine DTx-Plattform für entzündliche Erkrankungen auf den europäischen Märkten zu erweitern und gezielte Interventionen über Smartphones und Wearables zu ermöglichen. Solche Kooperationen unterstreichen das wachsende Vertrauen in DTx für die klinische Anwendung in der Praxis.
- Digitale Therapeutika bieten skalierbare, evidenzbasierte Lösungen, die Patienten Werkzeuge für Selbstmanagement, Verhaltensänderung und Echtzeit-Feedback an die Hand geben und so dazu beitragen, die Inanspruchnahme von Gesundheitsressourcen zu reduzieren.
- Der zunehmende Druck auf die Gesundheitssysteme, eine wertorientierte Versorgung zu gewährleisten, treibt die Einführung von DTx als wirksame Ergänzung oder Alternative zu konventionellen Behandlungen weiter voran
- Darüber hinaus ist DTx aufgrund der Flexibilität der Fernüberwachung, der Personalisierung und der automatisierten Fortschrittsverfolgung besonders für die alternde europäische Bevölkerung sowie für Menschen in unterversorgten oder ländlichen Regionen geeignet.
Einschränkung/Herausforderung
„Bedenken hinsichtlich des Datenschutzes und Hindernisse bei der Einhaltung gesetzlicher Vorschriften“
- Eine der größten Herausforderungen auf dem europäischen DTx-Markt besteht darin, die Einhaltung komplexer gesetzlicher Vorschriften und Datenschutzgesetze sicherzustellen, insbesondere der Datenschutz-Grundverordnung (DSGVO), die die Verwendung, Einwilligung und Sicherheit von Patientendaten regelt.
- So hat beispielsweise DiGA in Deutschland zwar den Marktzugang beschleunigt, erfordert aber die strikte Einhaltung von Datenschutzstandards und den Nachweis positiver Ergebnisse im Gesundheitswesen, was für aufstrebende Entwickler ressourcenintensiv sein kann.
- Patienten und Gesundheitsdienstleister zögern möglicherweise auch bei der Einführung von DTx-Lösungen, da sie Bedenken hinsichtlich Datenmissbrauchs, Schwachstellen in der Cybersicherheit und des Mangels an langfristigen Daten zur Wirksamkeit in der Praxis haben.
- Darüber hinaus kann die fragmentierte Regulierungslandschaft in den europäischen Ländern den Markteintritt und die Erstattungsabwicklung für Unternehmen, die über einen einzelnen nationalen Markt hinaus expandieren möchten, zu einer Herausforderung machen.
- Die Bewältigung dieser Herausforderungen durch transparente Datenpraktiken, fortschrittliche Verschlüsselung und die Generierung starker Beweise – kombiniert mit Harmonisierungsbemühungen der Gesundheitsbehörden auf EU-Ebene – wird für das langfristige Vertrauen und die Skalierbarkeit von DTx in ganz Europa von entscheidender Bedeutung sein.
Marktumfang für digitale Therapeutika (DTx) in Europa
Der Markt ist nach Produkt, Anwendung, Endbenutzer und Vertriebskanal segmentiert.
- Nach Produkt
Der europäische Markt für digitale Therapeutika (DTx) ist produktbezogen in Lösungen/Software, Hardwareprodukte und Dienstleistungen segmentiert. Das Segment Lösungen/Software dominierte den Markt mit dem größten Umsatzanteil von 65,2 % im Jahr 2024, was auf die einfache Bereitstellung, Kosteneffizienz und Skalierbarkeit bei der Bereitstellung personalisierter Interventionen über Apps und Cloud-Plattformen zurückzuführen ist. Softwarebasierte DTx werden häufig zur Behandlung chronischer Erkrankungen wie Diabetes und psychischer Erkrankungen eingesetzt, und zwar durch interaktive, KI-gesteuerte Therapiemodule und Echtzeit-Fortschrittsverfolgung.
Das Segment der Hardwareprodukte wird voraussichtlich von 2025 bis 2032 das höchste Wachstum verzeichnen. Dies wird durch die zunehmende Verbreitung tragbarer Geräte und sensorintegrierter Tools vorangetrieben, die die Patientenüberwachung verbessern und praxisnahe Gesundheitsdaten zur Unterstützung therapeutischer Ergebnisse liefern. Diese Produkte werden häufig in Verbindung mit Softwareplattformen eingesetzt, um eine kontinuierliche Patienteneinbindung und datengesteuerte Anpassungen zu gewährleisten.
- Nach Anwendung
Der europäische Markt für digitale Therapeutika (DTx) ist nach Anwendung in Behandlung, Prävention und Sonstiges unterteilt. Das Behandlungssegment hielt 2024 mit 58,3 % den größten Umsatzanteil, angetrieben durch die Nachfrage nach DTx zur Behandlung chronischer und psychischer Erkrankungen wie Diabetes, Bluthochdruck und Depression. Diese Plattformen bieten strukturierte, evidenzbasierte Therapien, die die Medikamenteneinnahmetreue, Verhaltensänderungen und die Fernverfolgung von Symptomen unterstützen.
Das Segment Prävention dürfte im Prognosezeitraum die höchste durchschnittliche jährliche Wachstumsrate (CAGR) verzeichnen. Dies wird durch die zunehmende Konzentration auf Lebensstilmanagement, frühzeitige Intervention und proaktive Strategien zur Einbindung der Gesundheit unterstützt, die die langfristigen Gesundheitskosten und das Fortschreiten der Krankheit reduzieren.
- Nach Endbenutzer
Der europäische Markt für digitale Therapeutika (DTx) ist nach Endnutzern in Krankenhäuser, Fachkliniken, ambulante Pflege und weitere Bereiche unterteilt. Krankenhäuser dominierten den Markt mit einem Umsatzanteil von 41,6 % im Jahr 2024, da DTx-Lösungen zunehmend in Krankenhausabläufe integriert werden, um die Genesung von Patienten, das Management chronischer Krankheiten und die Nachsorge zu unterstützen. Das institutionelle Vertrauen, die Verfügbarkeit von Erstattungsmöglichkeiten und der Fokus auf eine wertorientierte Versorgung machen Krankenhäuser zu wichtigen Anwendern digitaler Therapeutika.
Das Segment der häuslichen Gesundheitspflege wird im Prognosezeitraum voraussichtlich am stärksten wachsen, getrieben durch die steigende Nachfrage nach Fernversorgung, patientenzentrierten Modellen und die zunehmende Alterung der Bevölkerung. DTx-Lösungen ermöglichen es Patienten, ihre Beschwerden von zu Hause aus zu behandeln, wodurch Krankenhausaufenthalte reduziert und langfristige Pflegestrategien unterstützt werden.
- Nach Vertriebskanal
Der europäische Markt für digitale Therapeutika (DTx) ist nach Vertriebskanälen in Direktausschreibungen, Einzelhandelsverkäufe und andere segmentiert. Direktausschreibungen hatten 2024 mit 49,1 % den größten Umsatzanteil, getrieben durch groß angelegte Beschaffungen von Gesundheitsdienstleistern und -einrichtungen, insbesondere innerhalb nationaler Gesundheitssysteme und versicherungsfinanzierter Modelle in Ländern wie Deutschland und Großbritannien. Diese strukturierten Käufe ermöglichen den standardisierten Einsatz von DTx-Plattformen in Gesundheitseinrichtungen.
Der Einzelhandel wird im Prognosezeitraum voraussichtlich am stärksten wachsen, angetrieben durch die zunehmende Verfügbarkeit von DTx-Apps und -Plattformen über App Stores und Direktvertriebsmodelle. Das steigende Verbraucherbewusstsein und die zunehmenden Trends zur Selbstfürsorge ermutigen Einzelpersonen, selbstständig digitale Therapien anzuwenden, insbesondere für Lebensstil und psychische Erkrankungen.
Regionale Marktanalyse für digitale Therapeutika (DTx) in Europa
- Deutschland dominierte den europäischen Markt für digitale Therapeutika (DTx) mit dem größten Umsatzanteil von 31,7 % im Jahr 2024. Dies ist auf die Initiative für digitale Gesundheitsanwendungen (DiGA) zurückzuführen, die die Erstattung verschriebener DTx ermöglicht, auf ein unterstützendes regulatorisches Umfeld und eine starke Integration digitaler Therapeutika in klinische Behandlungspfade.
- Verbraucher und Gesundheitsdienstleister in der Region setzen zunehmend auf DTx-Lösungen aufgrund ihrer nachgewiesenen klinischen Wirksamkeit, des einfachen Fernzugriffs und der Fähigkeit, das langfristige Krankheitsmanagement, insbesondere bei chronischen und psychischen Erkrankungen, zu unterstützen.
- Diese zunehmende Akzeptanz wird durch die alternde Bevölkerung, den Ausbau der digitalen Gesundheitsinfrastruktur und politische Initiativen zur Senkung der Gesundheitskosten und Verbesserung der Patientenergebnisse weiter unterstützt. Digitale Therapeutika werden so zu einem Schlüsselelement in den zukünftigen europäischen Gesundheitsversorgungsmodellen.
Markteinblick in Europa für digitale Therapeutika (DTx)
Der europäische Markt für digitale Therapeutika (DTx) wird im Prognosezeitraum voraussichtlich deutlich wachsen. Dies wird durch die zunehmende Prävalenz chronischer Erkrankungen, die alternde Bevölkerung und die starke staatliche Förderung der digitalen Gesundheitsintegration vorangetrieben. Steigende Gesundheitskosten und der Trend zu einer wertorientierten Versorgung fördern die Einführung skalierbarer, evidenzbasierter digitaler Therapielösungen. Mit zunehmender regulatorischer Klarheit und in vielen Ländern etablierten Erstattungsmodellen gewinnen digitale Therapeutika in klinischen Behandlungspfaden sowohl zur Behandlung als auch zur Prävention chronischer und psychischer Erkrankungen an Bedeutung.
Markteinblick in Deutschland für digitale Therapeutika (DTx)
Die deutschen digitalen Therapeutika (DTx) erzielten 2024 mit 31,7 % den größten Umsatzanteil am europäischen DTx-Markt. Dies ist auf das bahnbrechende DiGA-Programm zurückzuführen, das die Erstattung verschriebener DTx durch die gesetzliche Krankenversicherung ermöglicht. Die starke digitale Gesundheitsinfrastruktur des Landes, kombiniert mit strengen Anforderungen an die klinische Validierung, fördert das Vertrauen von Gesundheitsdienstleistern und Patienten. Deutschlands führende Rolle bei regulatorischen Innovationen zieht globale und nationale DTx-Entwickler an und macht Deutschland zu einem zentralen Knotenpunkt für die Einführung digitaler Gesundheitsdienste in Europa.
Markteinblick in Großbritannien für digitale Therapeutika (DTx)
Der britische Markt für digitale Therapeutika (DTx) wird im Prognosezeitraum voraussichtlich mit einer robusten jährlichen Wachstumsrate wachsen, unterstützt durch den zunehmenden Einsatz digitaler Tools im National Health Service (NHS) zur Behandlung chronischer Erkrankungen und psychischer Probleme. Das Engagement der Regierung für eine digitale Versorgung und die umfassende Integration von Telemedizin und digitalen Plattformen positioniert Großbritannien als wichtigen Anwender von DTx. Die Zusammenarbeit zwischen öffentlichen Gesundheitsbehörden und privaten Entwicklern fördert zudem die Einführung klinisch validierter digitaler Therapien für verschiedene Patientengruppen.
Markteinblick in Frankreich für digitale Therapeutika (DTx)
Der französische Markt für digitale Therapeutika (DTx) entwickelt sich zu einem rasant wachsenden Markt. Beflügelt wird dies durch die Einführung nationaler Pilotprojekte zur Kostenerstattung wie PECAN, die auf die Integration digitaler Lösungen in die Standardbehandlung chronisch Kranker abzielen. Das landesweite Gesundheitssystem und hohe Investitionen in die digitale Transformation schaffen günstige Voraussetzungen für den Einsatz von DTx. Der zunehmende Fokus auf patientenzentrierte Versorgung und präventive Gesundheitsfürsorge beschleunigt die Einführung digitaler therapeutischer Interventionen, insbesondere in Bereichen wie Diabetes und Herz-Kreislauf-Erkrankungen.
Markteinblick für digitale Therapeutika (DTx) in Spanien
In Spanien wird aufgrund steigender Investitionen in Innovationen im Gesundheitswesen, des Ausbaus der digitalen Gesundheitsinfrastruktur und der zunehmenden Akzeptanz von Fernversorgungsmodellen ein stetiges Wachstum der digitalen Therapeutika (DTx) erwartet. Da das Gesundheitssystem nach kostengünstigen Lösungen für die Behandlung einer alternden Bevölkerung und chronischer Krankheiten sucht, gewinnen DTx-Plattformen zunehmend an Interesse. Lokale und regionale Gesundheitsbehörden testen zunehmend digitale Lösungen, insbesondere für das Diabetesmanagement, die psychische Unterstützung und die Änderung des Lebensstils.
Marktanteil digitaler Therapeutika (DTx) in Europa
Die europäische Digital Therapeutics (DTx)-Branche wird hauptsächlich von etablierten Unternehmen angeführt, darunter:
- Sidekick Health (Island)
- Kaia Health (Deutschland)
- HelloBetter (Deutschland)
- MySugr GmbH (Österreich)
- Voluntis SA (Frankreich)
- Resilient Digital Health (Frankreich)
- MediBioSense Ltd. (Großbritannien)
- SilverCloud Health (Großbritannien)
- Happify Health (Großbritannien)
- Zanadio von aidhere GmbH (Deutschland)
- TicTrac Ltd. (Großbritannien)
- Cureety (Frankreich)
- Oviva AG (Schweiz)
- Doccla Ltd. (Großbritannien)
- Psious (Spanien)
- S3 Connected Health (Irland)
- Qolware GmbH (Deutschland)
- Amicomed (Italien)
- Sooma Oy (Finnland)
Was sind die jüngsten Entwicklungen auf dem europäischen Markt für digitale Therapeutika (DTx)?
- Im März 2024 erweiterte Sidekick Health, ein in Island ansässiges Unternehmen für digitale Therapeutika, seine Partnerschaft mit Pfizer, um DTx-Programme für entzündliche Erkrankungen in mehreren europäischen Ländern, darunter Großbritannien und Deutschland, einzuführen. Der Schwerpunkt dieser Zusammenarbeit liegt auf der Bereitstellung evidenzbasierter digitaler Therapien für chronische Erkrankungen. Dies stärkt die Rolle von Sidekick Health bei der Integration von DTx in die allgemeine Gesundheitsversorgung und erweitert den Zugang zu personalisierten Pflegelösungen in ganz Europa.
- Im Februar 2024 erhielt HelloBetter, ein in Deutschland ansässiger DTx-Anbieter mit Spezialisierung auf psychische Gesundheit, die dauerhafte Kostenerstattung für mehrere seiner digitalen Therapieprogramme im Rahmen der DiGA. Dies stellt einen wichtigen Meilenstein in der deutschen Strategie für digitale Gesundheit dar, zeigt das wachsende institutionelle Vertrauen in digitale Therapeutika und schafft einen Präzedenzfall für die langfristige Integration von DTx in nationale Gesundheitssysteme.
- Im Januar 2024 gab Kaia Health mit Hauptsitz in Deutschland die Erweiterung seiner Plattform für muskuloskelettale (MSK) Schmerzbehandlung in Großbritannien und Frankreich bekannt. In Zusammenarbeit mit lokalen Gesundheitsdienstleistern soll die steigende Nachfrage nach nicht-invasiven, digitalen Therapien bedient werden. Die Initiative umfasst klinische Studien und die Generierung von Praxisbeweisen und unterstützt das übergeordnete Ziel des Unternehmens, ein führender Anbieter von MSK-Lösungen im europäischen Gesundheitsmarkt zu werden.
- Im Dezember 2023 brachte MediBioSense, ein britisches Unternehmen, das sich auf vernetzte Gesundheitstechnologien spezialisiert hat, eine tragbare Plattform mit DTx-Technologie auf den Markt, die sich an Herz- und Atemwegspatienten richtet. Das Gerät ist mit digitaler Therapiesoftware integriert, um kontinuierliche Überwachung und personalisierte Therapie zu ermöglichen und so dem wachsenden Bedarf an datenbasierter Fernversorgung im britischen National Health Service (NHS) gerecht zu werden.
- Im November 2023 stellte das französische Startup Resilient Digital Health ein vollständig digitales Diabetes-Management-Programm vor, das Verhaltensinterventionen mit Echtzeit-Glukosemessung und KI-gestütztem Coaching kombiniert. Das Programm wird in Zusammenarbeit mit öffentlichen Krankenhäusern in Paris erprobt und zielt darauf ab, die Wiedereinweisungen ins Krankenhaus zu reduzieren und die Patienteneinbindung durch digitale Versorgungsmodelle zu verbessern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE DIGITAL THERAPEUTICS (DTX) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT AND SERVICE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISING TECHNOLOGICAL ADVANCEMENTS IN DIGITAL HEALTHCARE DEVICES
5.1.2 INTEGRATION OF ARTIFICIAL INTELLIGENCE (AI) AND MACHINE LEARNING (ML)
5.1.3 INCREASING GOVERNMENT SUPPORT AND FUNDING
5.2 RESTRAINTS
5.2.1 DATA SECURITY AND PRIVACY CONCERNS RELATED TO DATA
5.2.2 HIGH LITERACY GAP IN DIGITAL HEALTH WITHIN THE POPULATION
5.3 OPPORTUNITIES
5.3.1 GROWING EXPANSION OF TELEHEALTH APPLICATIONS IN EUROPE
5.3.2 INCREASING FOCUS ON PERSONALIZED DEVICES FOR DIGITAL THERAPEUTICS
5.3.3 INCREASING STRATEGIC COLLABORATIONS AMONG THE MARKET PLAYERS
5.4 CHALLENGES
5.4.1 SOFTWARE INCOMPATIBILITY ISSUES DUE TO VARYING DATA STANDARDS
5.4.2 REGULATORY AND LEGAL CHALLENGES RELATED TO DTX DEVICES
6 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT
6.1 OVERVIEW
6.2 SOLUTIONS/SOFTWARE
6.2.1 MOBILE APPLICATIONS
6.2.2 WEB BASED PLATFORMS
6.2.3 ELECTRONIC HEALTH RECORDS
6.2.4 OTHERS
6.3 HARDWARE PRODUCTS
6.3.1 WEARABLE DEVICES
6.3.2 REMOTE PATIENT MONITORING DEVICES
6.4 SERVICES
7 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION
7.1 OVERVIEW
7.2 PREVENTIVE
7.2.1 OBESITY
7.2.2 PREDIABETES
7.2.3 OTHERS
7.3 TREATMENT
7.3.1 CARDIOVASCULAR
7.3.2 MENTAL HEALTH
7.3.3 DIABETES
7.3.4 RESPIRATORY CARE
7.4 OTHERS
8 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL
8.1 OVERVIEW
8.2 DIRECT TENDER
8.3 RETAIL SALES
8.4 OTHERS
9 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER
9.1 OVERVIEW
9.2 HOSPITALS
9.3 SPECIALTY CLINICS
9.4 HOME HEALTHCARE
9.5 OTHER
10 EUROPE DIGITAL THERAPEUTIC (DTX) MARKET, BY COUNTRY
10.1 EUROPE
10.1.1 GERMANY
10.1.2 FRANCE
10.1.3 ITALY
10.1.4 SPAIN
10.1.5 U.K.
10.1.6 SWITZERLAND
10.1.7 NETHERLANDS
10.1.8 RUSSIA
10.1.9 TURKEY
10.1.10 POLAND
10.1.11 SWEDEN
10.1.12 BELGIUM
10.1.13 DENMARK
10.1.14 FINLAND
10.1.15 NORWAY
10.1.16 REST OF EUROPE
11 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, COMPANY LANDSCAPE
11.1 COMPANY SHARE ANALYSIS: EUROPE
12 SWOT ANALYSIS
13 COMPANY PROFILE
13.1 HEALTHHERO
13.1.1 COMPANY SNAPSHOT
13.1.2 SERVICE PORTFOLIO
13.1.3 RECENT DEVELOPMENT
13.2 RESMED
13.2.1 COMPANY SNAPSHOT
13.2.2 REVENUE ANALYSIS
13.2.3 PRODUCT PORTFOLIO
13.2.4 RECENT DEVELOPMENT
13.3 GAIA AG
13.3.1 COMPANY SNAPSHOT
13.3.2 PRODUCT PORTFOLIO
13.3.3 RECENT DEVELOPMENT
13.4 SIDEKICK HEALTH GMBH
13.4.1 COMPANY SNAPSHOT
13.4.2 PRODUCT PORTFOLIO
13.4.3 RECENT DEVELOPMENT
13.5 KAIA HEALTH
13.5.1 COMPANY SNAPSHOT
13.5.2 PRODUCT PORTFOLIO
13.5.3 RECENT DEVELOPMENT
13.6 MINDABLE HEALTH GMBH
13.6.1 COMPANY SNAPSHOT
13.6.2 PRODUCT PORTFOLIO
13.6.3 RECENT DEVELOPMENT
14 QUESTIONNAIRE
15 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 2 EUROPE SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTIC (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 3 EUROPE HARDWARE PRODUCTS IN DIGITAL THERAPEUTIC (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 4 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 5 EUROPE PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 6 EUROPE TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 7 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 8 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 9 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY COUNTRY, 2022-2031 (USD MILLION)
TABLE 10 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 11 GERMANY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 12 GERMANY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 13 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 14 GERMANY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 15 GERMANY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 16 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 17 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 18 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 19 FRANCE SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 20 FRANCE HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 21 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 22 FRANCE PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 23 FRANCE TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 24 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 25 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 26 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 27 ITALY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 28 ITALY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 29 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 30 ITALY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 31 ITALY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 32 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 33 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 34 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 35 SPAIN SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 36 SPAIN HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 37 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 38 SPAIN PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 39 SPAIN TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 40 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 41 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 42 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 43 U.K. SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 44 U.K. HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 45 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 46 U.K. PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 47 U.K. TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 48 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 49 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 50 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 51 SWITZERLAND SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 52 SWITZERLAND HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 53 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 54 SWITZERLAND PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 55 SWITZERLAND TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 56 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 57 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 58 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 59 NETHERLANDS SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 60 NETHERLANDS HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 61 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 62 NETHERLANDS PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 63 NETHERLANDS TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 64 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 65 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 66 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 67 RUSSIA SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 68 RUSSIA HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 69 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 70 RUSSIA PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 71 RUSSIA TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 72 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 73 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 74 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 75 TURKEY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 76 TURKEY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 77 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 78 TURKEY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 79 TURKEY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 80 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 81 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 82 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 83 POLAND SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 84 POLAND HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 85 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 86 POLAND PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 87 POLAND TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 88 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 89 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 90 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 91 SWEDEN SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 92 SWEDEN HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 93 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 94 SWEDEN PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 95 SWEDEN TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 96 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 97 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 98 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 99 BELGIUM SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 100 BELGIUM HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 101 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 102 BELGIUM PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 103 BELGIUM TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 104 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 105 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 106 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 107 DENMARK SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 108 DENMARK HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 109 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 110 DENMARK PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 111 DENMARK TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 112 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 113 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 114 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 115 FINLAND SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 116 FINLAND HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 117 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 118 FINLAND PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 119 FINLAND TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 120 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 121 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 122 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 123 NORWAY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 124 NORWAY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 125 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 126 NORWAY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 127 NORWAY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 128 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 129 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 130 REST OF EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
Abbildungsverzeichnis
FIGURE 1 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: SEGMENTATION
FIGURE 2 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 10 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: SEGMENTATION
FIGURE 12 RISING TECHNOLOGICAL ADVANCEMENTS IN THE DISEASE DIAGNOSIS AND TREATMENT APPROACH, INCREASING INVESTMENT IN EARLY STAGE VENTURES, REIMBURSEMENT COVERAGE PROVIDED BY THE REGULATORY AGENCIES ARE SOME OF THE FACTORS EXPECTED TO DRIVE THE EUROPE DIGITAL THERAPEUTICS MARKET IN THE FORECAST PERIOD 2024 TO 2031
FIGURE 13 SOLUTIONS/ SOFTWARES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE DIGITAL THERAPEUTICS (DTX) MARKET IN 2024 AND 2031
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE DIGITAL THERAPEUTICS (DTX) MARKET
FIGURE 15 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, 2023
FIGURE 16 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, 2024-2031 (USD MILLION)
FIGURE 17 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, CAGR (2024-2031)
FIGURE 18 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 19 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, 2023
FIGURE 20 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, 2024-2031 (USD MILLION)
FIGURE 21 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, CAGR (2024-2031)
FIGURE 22 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 23 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, 2023
FIGURE 24 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, 2024-2031 (USD MILLION)
FIGURE 25 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2024-2031)
FIGURE 26 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 27 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, 2023
FIGURE 28 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, 2024-2031 (USD MILLION)
FIGURE 29 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, CAGR (2024-2031)
FIGURE 30 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: SNAPSHOT (2023)
FIGURE 32 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: COMPANY SHARE 2023 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.