Europe Automated External Defibrillator Aed Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.29 Billion
USD
1.95 Billion
2024
2032
USD
1.29 Billion
USD
1.95 Billion
2024
2032
| 2025 –2032 | |
| USD 1.29 Billion | |
| USD 1.95 Billion | |
|
|
|
|
Europe Automated External Defibrillator (AED) Market Segmentation, By Product Type (Semi-Automated External Defibrillators, and Fully-Automated External Defibrillators), End-User (Pre-hospitals, Public Access Facilities, Hospitals, Alternate Care, and Home) - Industry Trends and Forecast to 2032
Europe Automated External Defibrillator (AED) Market Size
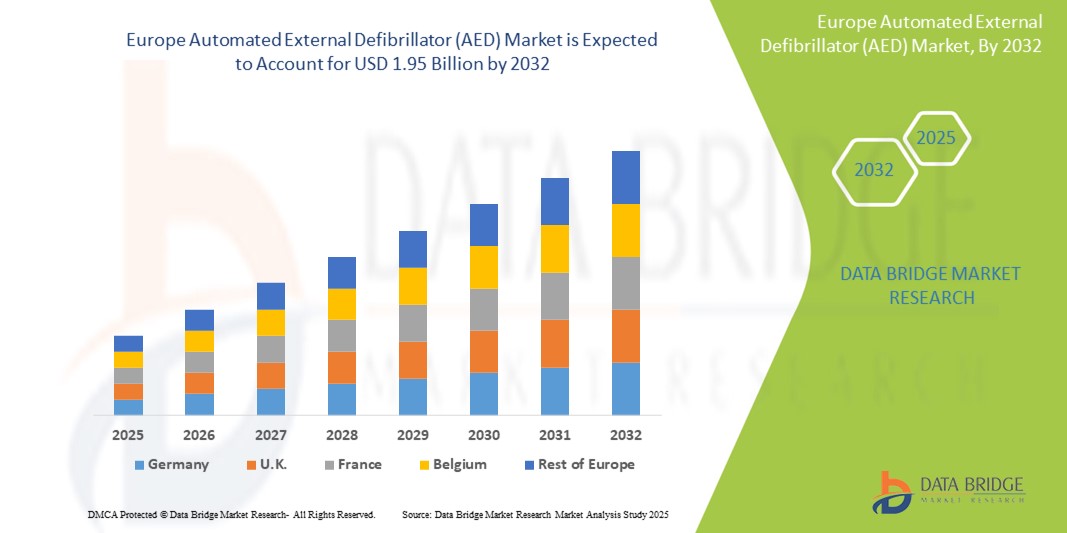
- The Europe automated external defibrillator (AED) market size was valued atUSD 1.29 billion in 2024and is expected to reachUSD 1.95 billion by 2032, at aCAGR of 6.14%during the forecast period
- This growth is driven by factors such as the increasing prevalence of cardiovascular diseases, rising awareness about the importance of early defibrillation, advancements in AED technology, and growing adoption of AEDs in public places, workplaces, and healthcare facilities to enhance emergency response capabilities
Europe Automated External Defibrillator (AED) Market Analysis
- The automated external defibrillator market in Europe is growing rapidly as awareness of sudden cardiac arrests increases. For instance, many cities are now installing public access defibrillators in high-traffic areas.
- Technological advancements have made defibrillators more efficient and user-friendly. These improvements have encouraged wider adoption in locations such as airports, schools, and sports venues
- The U.K. is expected to dominate the market with a market share of 6.9%, supported by a well-established healthcare infrastructure that enables widespread AED deployment and utilization
- Germany’s is expected to be the fastest growing country, supported by multiple key factor including substantial government healthcare spending, a robust healthcare infrastructure, and a growing elderly population at elevated risk for cardiovascular conditions
- The hospital segment is expected to dominate the market with the largest share of 26.06% in 2025 due to the increasing adoption of AEDs in medical facilities for rapid response to cardiac emergencies. Hospitals are prioritizing patient safety by equipping their premises with these life-saving devices across various departments, including emergency rooms, surgical areas, and intensive care units
Report Scope and Europe Automated External Defibrillator (AED) Market Segmentation
|
Attributes |
Europe Automated External Defibrillator (AED) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Europe Automated External Defibrillator (AED) Market Trends
“Rise of Smart Connectivity in Defibrillator Devices”
- Smart connectivity is becoming a key trend in automated external defibrillators, making devices easier to monitor and manage remotely
- Devices now offer real-time data sharing with emergency teams, helping responders act faster during critical situations
- Many modern defibrillators are linked to cloud systems, allowing regular updates, performance checks, and maintenance alerts
- For instance, some defibrillators can alert users and service providers if the battery is low or the device needs servicing
- In conclusion, as more healthcare providers focus on faster emergency response, the demand for connected defibrillators continues to grow rapidly
Europe Automated External Defibrillator (AED) Market Dynamics
Driver
“Growing Public Awareness of Sudden Cardiac Arrest Response”
- Public awareness of sudden cardiac arrest is rising through health campaigns and training programs such as those by the British Heart Foundation and European Resuscitation Council
- Training in cardiopulmonary resuscitation and defibrillator use is increasingly provided in schools, workplaces, and community centers such as through St John Ambulance courses
- Demand for defibrillators in public areas is growing with installations in locations such as Heathrow Airport, London Underground stations, and major football stadiums
- For instance, a man’s life was saved at London’s King’s Cross station in 2023 when bystanders used a public defibrillator before paramedics arrived
- This growing exposure and successful real-life usage are encouraging both individuals and organizations to invest in wider defibrillator access
- In conclusion, awareness and public readiness are directly contributing to market expansion through greater community-level defibrillator adoption
Opportunity
“Expansion of Defibrillator Installations in Workplaces”
- Companies are increasingly prioritizing workplace safety by installing defibrillators as part of their health and emergency response policies
- This opportunity extends to diverse settings such as offices, factories, warehouses, and remote job sites where employee safety is a growing concern
- For instance, Amazon has equipped several of its European warehouses with defibrillators and offers staff training as part of its health initiative
- Some governments, such as in France and Germany, are encouraging workplace defibrillator adoption through safety regulations and corporate responsibility guidelines
- As more employers include defibrillator training in onboarding and wellness programs, the corporate sector is becoming a key market driver
- In conclusion, the workplace sector presents a valuable and expanding opportunity for defibrillator integration across industries and locations
Restraint/Challenge
“High Cost of Device Maintenance and Replacement”
- The ongoing costs of maintaining automated external defibrillators, such as battery and electrode pad replacements, create a significant financial burden for many organizations
- For instance, small community centers or remote job sites may struggle to afford the upkeep, preventing them from investing in defibrillator units
- In environments with fluctuating conditions, defibrillators are more prone to damage or wear, further escalating maintenance costs for protective casings or device replacement
- Without consistent funding or proper maintenance protocols, some defibrillators may remain out of service, leaving them unusable in emergencies when most needed
- Lack of awareness and training on maintenance practices among users often results in devices being overlooked or neglected, reducing their effectiveness
- In conclusion, the high cost and complexity of defibrillator maintenance hinder widespread adoption and reliable functionality, limiting market growth
Europe Automated External Defibrillator (AED) Market Scope
The market is segmented on the basis of product type and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By End User |
|
In 2025, the hospital segment is projected to dominate the market with a largest share in end user segment
The hospital segment is expected to dominate the Europe automated external defibrillator (AED) market with the largest share of 26.06% in 2025 due to the increasing adoption of AEDS in medical facilities for rapid response to cardiac emergencies. Hospitals are prioritizing patient safety by equipping their premises with these life-saving devices across various departments, including emergency rooms, surgical areas, and intensive care units. The demand for AEDS is also driven by hospital protocols that require the availability of defibrillators in case of sudden cardiac arrest. Additionally, hospitals are investing in advanced, user-friendly AED models that allow medical staff to provide quick and effective treatment.
The semi-automated external defibrillators segment is expected to account for the largest share during the forecast period in product type segment
In 2025, the semi-automated external defibrillators segment is expected to dominate the market with the largest market share of 25.11% due to their ease of use and affordability compared to fully automated models. These devices are designed to guide users through the defibrillation process with audible prompts, making them suitable for both trained and untrained individuals. Their semi-automated nature provides a balance between automation and manual intervention, which appeals to many public facilities, workplaces, and educational institutions.
Europe Automated External Defibrillator (AED) Market Regional Analysis
“Germany Holds the Largest Share in the Europe Automated External Defibrillator (AED) Market”
- The U.K. is expected to dominate the market with a market share of 6.9%, supported by a well-established healthcare infrastructure that enables widespread AED deployment and utilization
- The strong presence of key industry players in the country further reinforces its market dominance
- In addition, the rising prevalence of cardiovascular diseases and an aging population continue to fuel demand for AEDs, firmly positioning the U.K. as a frontrunner in AED adoption across Europe
“Germany is Projected to Register the Highest CAGR in the Europe Automated External Defibrillator (AED) Market”
- Germany’s is expected to be the fastest growing country, supported by multiple key factor including substantial government healthcare spending, a robust healthcare infrastructure, and a growing elderly population at elevated risk for cardiovascular conditions
- In addition, the local presence of leading medical device companies and strict workplace safety regulations further contribute to market expansion
- German employers increasingly view AEDs as essential safety equipment, integrating them into workplace health and safety protocols to safeguard employee well-being
Europe Automated External Defibrillator (AED) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Koninklijke Philips N.V. (Netherlands)
- GE Healthcare (U.K.)
- Siemens Healthineers AG (Germany)
- Hillrom Holdings, Inc. (U.K.)
- Schiller AG (Switzerland)
- Fukuda Denshi (Japan)
- BPL Medical Technologies (India)
- Medtronic plc (U.S.)
- Asahi Kasei Corporation (Japan)
- NIHON KOHDEN CORPORATION (Japan)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- Stryker (U.S.)
- CU Medical Germany GmbH (Germany)
- MEDIANA Co., Ltd. (South Korea)
- Defibtech LLC (Germany)
- Cardiac Science Corporation (U.K.)
- HeartSine Technologies (U.K.)
- ZOLL Medical Corporation (U.K.)
- Physio-Control (U.K.)
Latest Developments in Europe Automated External Defibrillator (AED) Market
- In July 2023, The U.K. government launched CellAED, the world's first personal AED, designed to be ultra-portable and user-friendly, in collaboration with the NHS supply chain, aiming to make AEDs more accessible and affordable for consumers
- In January 2023, Virginia Tech University, with assistance from the Virginia Tech Police Department and Environmental Health and Safety, implemented an AED program across the Blacksburg campus, managed by the Virginia Tech Rescue Squad, to enhance emergency response capabilities and support cardiac arrest victims
- In September 2022, Kauvery Hospital launched the Restart Heart Foundation, installing voice-prompted AEDs aimed at providing life-saving support during sudden cardiac arrests, contributing to improved emergency response in healthcare practices
- In August 2022, Portage Health Foundation initiated a one-time grant program for automated external defibrillators (AEDs) to enhance community safety as part of its long-term sustainability goals
- In January 2022, Euroa, Australia, achieved Heart-Safe Community status by installing 10 defibrillators, 6 of which are accessible 24/7, and training residents in emergency response, aiming to boost cardiac arrest survival rates in Victoria
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.