North America Molecular Diagnostics Services Market
市场规模(十亿美元)
CAGR :
% 
 USD
56,705.30 Thousand
USD
103,058.75 Thousand
2022
2030
USD
56,705.30 Thousand
USD
103,058.75 Thousand
2022
2030
| 2023 –2030 | |
| USD 56,705.30 Thousand | |
| USD 103,058.75 Thousand | |
|
|
|
|
北美分子诊断服务市场,按服务类型(仪器维修服务、培训服务、合规服务、校准服务维护服务、可扩展自动化服务、交钥匙服务、仪器搬迁服务、硬件定制、性能保证服务、设计和开发服务、供应链解决方案、新产品介绍服务、制造服务、环境和监管服务、医疗管理系统认证和审计、临床研究服务、咨询服务和其他服务)、技术(PCR、实时 PCR、下一代测序和其他技术)、最终用户(医院、诊断中心、学术和研究机构等)行业趋势和预测到 2030 年。
北美分子诊断服务市场分析与洞察
对检测、治疗和更快诊断的需求不断增长,有助于促进整体市场增长。分子诊断领域的资金不断增加也促进了市场的增长。主要市场参与者高度关注各种新的诊断服务。此外,慢性病和传染病的发病率不断上升也推动了市场需求的上升。


由于市场参与者的增加以及市场上各种先进分子诊断服务的可用性,北美分子诊断服务市场在预测年内将增长。与此同时,市场上政府和私人合作的数量也有所增加,这进一步推动了市场增长。然而,与分子诊断实验室设置和其他仪器相关的高成本可能会阻碍预测期内的市场增长。
主要市场参与者的战略举措、政府和私人合作的不断增加以及研发活动的不断增加为市场增长提供了机会。然而,政府的严格监管和缺乏熟练的专业人员是市场增长的主要挑战。
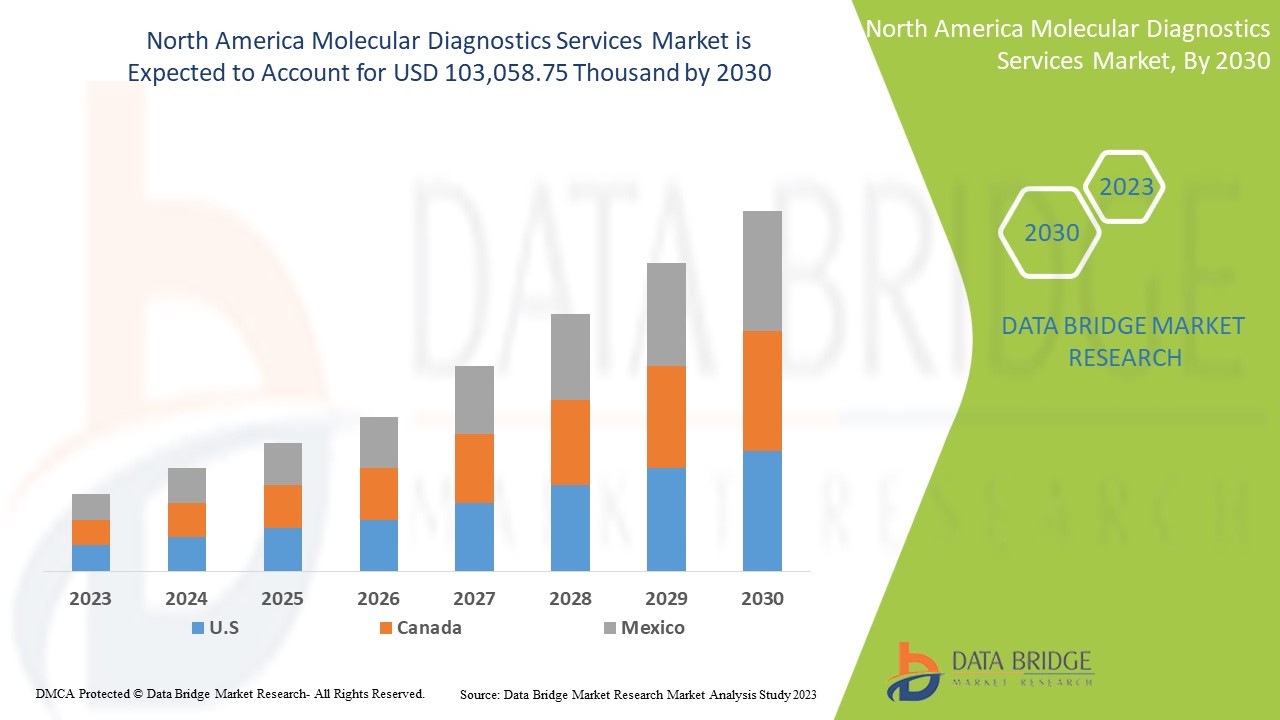
北美分子诊断服务市场预计将从 2022 年的 56,705.30 万美元增至 2030 年的 103,058.75 万美元,在 2023 年至 2030 年的预测期内以 8.0% 的复合年增长率增长。
|
报告指标 |
细节 |
|
预测期 |
2023 至 2030 年 |
|
基准年 |
2022 |
|
历史年份 |
2021(可定制为 2020 - 2016) |
|
定量单位 |
收入(千美元) |
|
涵盖的领域 |
按服务类型(仪器维修服务、培训服务、合规服务、校准服务、维护服务、可扩展自动化服务、交钥匙服务、仪器搬迁服务、硬件定制、性能保证服务、设计和开发服务、供应链解决方案、新产品介绍服务、制造服务、环境和监管服务、医疗管理系统认证和审计、临床研究服务、咨询服务和其他服务)、技术(PCR、实时 PCR、下一代测序和其他技术)、最终用户(医院、诊断中心、学术和研究机构等)。 |
|
覆盖国家 |
美国、加拿大和墨西哥 |
|
涵盖的市场参与者 |
F. Hoffmann-La Roche Ltd、Danaher、BIOMERIEUX、QIAGEN、Thermo Fisher Scientific Inc.、Bio-Rad Laboratories, Inc、Abbott、DiaSorin SpA 和 Hologic, Inc 等 |
市场定义
分子诊断是通过研究组织或体液中的蛋白质、DNA 和 RNA 等分子来识别疾病的过程。这些分子诊断服务是一种策略,借助实验室技术来研究、分析和诊断某些分子。它们专门用于测量体液中的特定生物标志物、基于聚合酶链反应 (PCR) 的细菌基因检测以及任何特定感染的分子反应。这些服务用于提供更有效的测试和诊断。
分子诊断服务使研究人员和技术人员能够在更短的时间内高效地完成工作,从而推动了亚太地区分子诊断服务市场的发展。此外,由于具有临床实践的潜力,这些服务可用于提供额外支持以提高分子诊断的效率,主要市场参与者的存在也促进了市场的增长。
北美分子诊断服务市场动态
驱动程序
-
慢性病和传染病发病率上升
目前,慢性病和传染病是几乎所有国家成年人死亡的主要原因。慢性病和传染病的发病率正在上升,因此人们更加依赖诊断实验室和疾病诊断中心。发病率的上升增加了对分子诊断服务的需求,以便在更短的时间内提供准确的诊断、治疗和检测结果。有害健康的行为——尤其是吸烟、缺乏体育锻炼、不良饮食习惯和过量饮酒——是导致慢性病和传染病发病率上升的主要原因。这些疾病可以通过多种技术进行诊断,例如免疫测定、分子技术、PCR 和基因操作技术等。因此,对分子诊断服务的需求正在增加,以满足患者对更快诊断和治疗的需求。
慢性病和传染病的发病率很高,诊断和治疗所需的分子检测数量也增加了,对产品和服务的需求最终将会增加。因此,慢性病和传染病的发病率上升正在推动全球市场的增长。
-
对检测、治疗和快速诊断的需求不断增加
目前,对高价专科治疗的需求更大。消费者对快速诊断的认识正在提高,分子诊断分析软件的采用率正在以更高的速度增长。分子诊断提供准确有效的结果,在疾病诊断中具有不可或缺的应用。人们更喜欢依赖于识别个人的遗传、表观基因组和临床信息的个性化药物。
此外,近年来,患者正在转向个性化的护理和治疗,对分子检测、治疗和更快诊断的需求正在增加,这可能成为全球市场增长的驱动因素。
-
分子诊断领域的资金不断增加
政府为支持和促进创新和发展而采取的举措、计划和资助活动不断增加,因此,分子诊断领域正在迅速发展。该领域是疾病控制不可或缺的一部分,因此资金不断增加,科学家也更加专注于开发疾病控制和健康生存的新思路。癌症等慢性病再次成为全球的主导,对专业治疗和最快诊断的需求正在上升。
随着政府和公共资金不断增加,以支持分子诊断领域,以实现更好的创新和更快诊断的替代方案,市场正在更大程度上得到推动。考虑到上述情况,可以预测,在不久的将来,分子诊断领域的资金增长将推动市场发展。
机会
-
增加研发活动
日益增多的研发活动为全球范围内获取分子诊断服务提供了替代选择,提高了可及性并降低了诊断服务相关成本。然而,由于进展缓慢且分散,分子诊断的全部潜力尚未得到充分发挥。
意识计划的增加、意识倡议的提高以及政府的支持增加了分子诊断在各个领域的使用。增加的研发活动将有助于增加开发新产品或改进产品和工艺的知识。设计、技术制造和阶段试验通常是研发活动中最重要的部分,可以改善服务。
随着市场参与者不断从事研发活动,更多的分子诊断服务可能会进入市场。这将增加医院、诊所和诊断中心等各种终端用户对分子诊断服务的需求。
克制/挑战
- 分子诊断实验室设置和其他仪器的成本高昂
分子诊断实验室包括多种技术,例如荧光原位杂交 (FISH)、基于聚合酶链反应 (PCR) 的分子诊断、间期染色体分析、DNA 指纹识别等。分子诊断实验室提供多种服务,例如安装服务、验证服务、整改服务、自动化服务等。因此,整体实验室设置和仪器成本过高,如果没有政府和私人资金和投资,很难建立,这可能会抑制市场增长。
COVID-19 对北美分子诊断服务市场的影响
后新冠疫情对市场产生了积极影响。大量人口受到此次疫情的影响,全球诊断测试数量有所增加。大多数情况下,基于 PCR 的 COVID-19 诊断有所增加。因此,COVID-19对市场产生了积极影响。
近期发展
- 2022 年 8 月,雅培分享了一项关于脑震荡血液检测的新研究,该检测可以预测脑损伤的结果并为治疗干预提供信息。这有助于该公司扩大其在北美市场的影响力。
北美分子诊断服务市场范围
北美分子诊断服务市场根据服务类型、技术和最终用户分为三个显著的细分市场。这些细分市场之间的增长将帮助您分析行业中增长微弱的细分市场,并为用户提供有价值的市场概览和市场洞察,以便做出战略决策,确定核心市场应用。
按服务类型
- 仪器维修服务
- 培训服务
- 维护服务
- 校准服务
- 合规服务
- 交钥匙服务
- 可扩展自动化服务
- 硬件定制
- 仪器搬迁服务
- 绩效保证服务
- 设计与开发服务
- 供应链解决方案
- 新产品介绍服务
- 制造服务
- 环境及监管服务
- 医疗管理系统认证与审核
- 临床研究服务
- 咨询服务
- 其他服务
根据服务类型,市场细分为仪器维修服务、培训服务、维护服务、校准服务、合规服务、交钥匙服务、可扩展自动化服务、硬件定制、仪器搬迁服务、性能保证服务、设计和开发服务、供应链解决方案、新产品介绍服务、制造服务、环境和监管服务、医疗管理系统认证和审计、临床研究服务、咨询服务和其他服务。
按技术分类
- 聚合酶链反应
- 实时 PCR
- 下一代测序
- 其他技术
根据技术,市场细分为 PCR、实时 PCR、下一代测序和其他技术。
按最终用户
- 医院
- 诊断中心
- 学术及研究机构
- 其他的

根据最终用户,市场分为医院、诊断中心、学术和研究机构等。
北美分子诊断服务市场区域分析/见解
对北美分子诊断服务市场进行了分析,并按国家、服务类型、技术和最终用户提供了市场规模洞察和趋势。
本报告涉及的国家包括美国、加拿大和墨西哥。
美国在市场份额和市场收入方面占据北美分子诊断服务市场的主导地位,并将在预测期内继续保持其主导地位。
这是由于在人们中实施了意识计划并且技术进步的进一步促进了这一市场的增长。
报告的国家部分还提供了影响单个市场因素和市场法规变化的信息,这些因素和变化会影响市场的当前和未来趋势。新旧销售、国家人口统计、疾病流行病学和进出口关税等数据点是预测单个国家市场情况的一些主要指标。此外,在对国家数据进行预测分析时,还考虑了北美品牌的存在和可用性、当地和国内品牌激烈竞争所带来的挑战以及销售渠道的影响。
竞争格局和北美分子诊断服务市场份额分析
北美分子诊断服务市场竞争格局提供了竞争对手的详细信息。详细信息包括公司概况、公司财务状况、收入、市场潜力、研发投资、新市场计划、北美业务、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度以及应用主导地位。以上提供的数据点仅与公司对市场的关注有关。
北美分子诊断服务市场的一些主要参与者包括 F. Hoffmann-La Roche Ltd、Danaher、BIOMERIEUX、QIAGEN、Thermo Fisher Scientific Inc.、Bio-Rad Laboratories, Inc、Abbott、DiaSorin SpA 和 Hologic, Inc 等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISING PREVALENCE OF CHRONIC AND INFECTIOUS DISEASES
5.1.2 INCREASING DEMAND FOR TESTING, THERAPIES, AND FASTER DIAGNOSTICS
5.1.3 GROWING FUNDINGS IN MOLECULAR DIAGNOSTICS SEGMENT
5.2 RESTRAINTS
5.2.1 HIGH-COST ASSOCIATED WITH MOLECULAR DIAGNOSTICS LABS SET-UPS AND OTHER INSTRUMENTATIONS
5.2.2 NON-AVAILABILITY OF RELEVANT AND APPROPRIATE KITS
5.3 OPPORTUNITIES
5.3.1 STRATEGIC INITIATIVES BY KEY PLAYERS
5.3.2 RISING GOVERNMENT AND PRIVATE COLLABORATIONS
5.3.3 INCREASING RESEARCH AND DEVELOPMENT ACTIVITIES
5.4 CHALLENGES
5.4.1 STRINGENT REGULATIONS
5.4.2 LACK OF SKILLED PROFESSIONALS
6 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE
6.1 OVERVIEW
6.2 INSTRUMENT REPAIR SERVICES
6.2.1 ON-SITE REPAIR SERVICES
6.2.1 OFF-SITE REPAIR SERVICES
6.3 MAINTENANCE SERVICES
6.4 TRAINING SERVICES
6.4.1 PCR BASED MOLECULAR DIAGNOSTICS
6.4.2 IMMUNOHISTOCHEMISTRY (IHC)
6.4.3 VIRAL LOAD TESTING
6.4.4 INTERPHASE CHROMOSOME PROFILING (ICP)
6.4.5 APPLICATION TRAINING SERVICES
6.5 CALIBRATION SERVICES
6.6 COMPLIANCE SERVICES
6.6.1 IQ/OQ & PM/OQ SERVICES
6.6.2 INSTALLATION SERVICES
6.6.3 VALIDATION SERVICES
6.6.4 OTHER SERVICES
6.7 TURNKEY SERVICES
6.8 SCALABLE AUTOMATION SERVICES
6.8.1 AUTOMATION FOR HIGH-CONTENT SCREENING (HCS)
6.8.2 AUTOMATION FOR HIGH-THROUGHPUT PLATE-BASED ASSAYS
6.8.3 AUTOMATION FOR HIGH-THROUGHPUT CLONE SCREENING
6.9 HARDWARE CUSTOMIZATION
6.1 INSTRUMENT RELOCATION SERVICES
6.10.1 PLATE RECERTIFICATION
6.10.2 FACTORY-APPROVED PARTS
6.10.3 INSTRUMENT PERFORMANCE EVALUATION
6.10.4 OTHER SERVICES
6.11 PERFORMANCE ASSURANCE SERVICES
6.12 DESIGN AND DEVELOPMENT SERVICES
6.13 SUPPLY CHAIN SOLUTIONS
6.14 NEW PRODUCT INTRODUCTION SERVICES
6.15 MANUFACTURING SERVICES
6.16 ENVIRONMENTAL AND REGULATORY SERVICES
6.17 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION & AUDITING
6.18 CLINICAL RESEARCH SERVICES
6.19 CONSULTATIVE SERVICES
6.2 OTHER SERVICES
7 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY TECHNOLOGY
7.1 OVERVIEW
7.2 REAL TIME PCR
7.2.1 INSTRUMENT REPAIR SERVICES
7.2.2 MAINTENANCE SERVICES
7.2.3 TRAINING SERVICES
7.2.4 CALIBRATION SERVICES
7.2.5 COMPLIANCE SERVICES
7.2.6 TURNKEY SERVICES
7.2.7 SCALABLE AUTOMATION SERVICES
7.2.8 HARDWARE CUSTOMIZATION
7.2.9 INSTRUMENT RELOCATION SERVICES
7.2.10 PERFORMANCE ASSURANCE SERVICES
7.2.11 DESIGN AND DEVELOPMENT SERVICES
7.2.12 SUPPLY CHAIN SOLUTIONS
7.2.13 NEW PRODUCT INTRODUCTION SERVICES
7.2.14 MANUFACTURING SERVICES
7.2.15 ENVIRONMENTAL & REGULATION SERVICES
7.2.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION & AUDITING
7.2.17 CLINICAL RESEARCH SERVICES
7.2.18 CONSULTATIVE SERVICES
7.2.19 OTHER SERVICES
7.3 PCR
7.3.1 INSTRUMENT REPAIR SERVICES
7.3.2 MAINTENANCE SERVICES
7.3.3 TRAINING SERVICES
7.3.4 CALIBRATION SERVICES
7.3.5 COMPLIANCE SERVICES
7.3.6 TURNKEY SERVICES
7.3.7 SCALABLE AUTOMATION SERVICES
7.3.8 HARDWARE CUSTOMIZATION
7.3.9 INSTRUMENT RELOCATION SERVICES
7.3.10 PERFORMANCE ASSURANCE SERVICES
7.3.11 DESIGN AND DEVELOPMENT SERVICES
7.3.12 SUPPLY CHAIN SOLUTIONS
7.3.13 NEW PRODUCT INTRODUCTION SERVICES
7.3.14 MANUFACTURING SERVICES
7.3.15 ENVIRONMENTAL AND REGULATION SERVICES
7.3.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION & AUDITING
7.3.17 CLINICAL RESEARCH SERVICES
7.3.18 CONSULTATIVE SERVICES
7.3.19 OTHER SERVICES
7.4 NEXT GENERATION SEQUENCING
7.4.1 INSTRUMENT REPAIR SERVICES
7.4.2 MAINTENANCE SERVICES
7.4.3 TRAINING SERVICES
7.4.4 CALIBRATION SERVICES
7.4.5 COMPLIANCE SERVICES
7.4.6 TURNKEY SERVICES
7.4.7 SCALABLE AUTOMATION SERVICES
7.4.8 HARDWARE CUSTOMIZATION
7.4.9 INSTRUMENT RELOCATION SERVICES
7.4.10 PERFORMANCE ASSURANCE SERVICES
7.4.11 DESIGN AND DEVELOPMENT SERVICES
7.4.12 SUPPLY CHAIN SOLUTIONS
7.4.13 NEW PRODUCT INTRODUCTION SERVICES
7.4.14 MANUFACTURING SERVICES
7.4.15 ENVIRONMENTAL AND REGULATION SERVICES
7.4.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION & AUDITING
7.4.17 CLINICAL RESEARCH SERVICES
7.4.18 CONSULTATIVE SERVICES
7.4.19 OTHER SERVICES
7.5 OTHER TECHNOLOGIES
8 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER
8.1 OVERVIEW
8.2 HOSPITALS
8.2.1 INSTRUMENT REPAIR SERVICES
8.2.2 MAINTENANCE SERVICES
8.2.3 TRAINING SERVICES
8.2.4 CALIBRATION SERVICES
8.2.5 COMPLIANCE SERVICES
8.2.6 TURNKEY SERVICES
8.2.7 SCALABLE AUTOMATION SERVICES
8.2.8 HARDWARE CUSTOMIZATION
8.2.9 INSTRUMENT RELOCATION SERVICES
8.2.10 PERFORMANCE ASSURANCE SERVICES
8.2.11 DESIGN AND DEVELOPMENT SERVICES
8.2.12 SUPPLY CHAIN SOLUTIONS
8.2.13 NEW PRODUCT INTRODUCTION SERVICES
8.2.14 MANUFACTURING SERVICES
8.2.15 ENVIRONMENTAL AND REGULATORY SERVICES
8.2.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION AND AUDITING
8.2.17 CLINICAL RESEARCH SERVICES
8.2.18 CONSULTATIVE SERVICES
8.2.19 OTHER SERVICES
8.3 DIAGNOSTIC CENTERS
8.3.1 CLINICAL LABORATORIES
8.3.1.1 INSTRUMENT REPAIR SERVICES
8.3.1.2 Maintenance services
8.3.1.3 Training SERVICES
8.3.1.4 CALIBRATION SERVICES
8.3.1.5 COMPLIANCE SERVICES
8.3.1.6 TURNKEY SERVICES
8.3.1.7 SCALABLE AUTOMATION SERVICES
8.3.1.8 HARDWARE CUSTOMIZATION
8.3.1.9 INSTRUMENT RELOCATION SERVICES
8.3.1.10 PERFORMANCE Assurance SERVICES
8.3.1.11 DESIGN and DEVELOPMENT SERVICES
8.3.1.12 SUPPLY CHAIN SOLUTIONS
8.3.1.13 NEW PRODUCT INTRODUCTION SERVICES
8.3.1.14 MANUFACTURING SERVICES
8.3.1.15 ENVIRONMENTAL and REGULATory SERVICES
8.3.1.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION and AUDITING
8.3.1.17 CLINICAL RESEARCH SERVICES
8.3.1.18 CONSULTATIVE SERVICES
8.3.1.19 Other SERVICES
8.3.2 AMBULATORY SURGICAL CENTERS
8.3.2.1 INSTRUMENT REPAIR SERVICES
8.3.2.2 MAINTENANCE SERVICES
8.3.2.3 TRAINING SERVICES
8.3.2.4 CALIBRATION SERVICES
8.3.2.5 COMPLIANCE SERVICES
8.3.2.6 TURNKEY SERVICES
8.3.2.7 SCALABLE AUTOMATION SERVICES
8.3.2.8 HARDWARE CUSTOMIZATION
8.3.2.9 INSTRUMENT RELOCATION SERVICES
8.3.2.10 PERFORMANCE Assurance SERVICES
8.3.2.11 DESIGN and DEVELOPMENT SERVICES
8.3.2.12 SUPPLY CHAIN SOLUTIONS
8.3.2.13 NEW PRODUCT INTRODUCTION SERVICES
8.3.2.14 MANUFACTURING SERVICES
8.3.2.15 ENVIRONMENTAL AND REGULATORY SERVICES
8.3.2.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION and AUDITING
8.3.2.17 CLINICAL RESEARCH SERVICES
8.3.2.18 CONSULTATIVE SERVICES
8.3.2.19 OTHER SERVICES
8.4 ACADEMIC AND RESEARCH INSTITUTES
8.4.1 INSTRUMENT REPAIR SERVICES
8.4.2 MAINTENANCE SERVICES
8.4.3 TRAINING SERVICES
8.4.4 CALIBRATION SERVICES
8.4.5 COMPLIANCE SERVICES
8.4.6 TURNKEY SERVICES
8.4.7 SCALABLE AUTOMATION SERVICES
8.4.8 HARDWARE CUSTOMIZATION
8.4.9 INSTRUMENT RELOCATION SERVICES
8.4.10 PERFORMANCE ASSURANCE SERVICES
8.4.11 DESIGN AND DEVELOPMENT SERVICES
8.4.12 SUPPLY CHAIN SOLUTIONS
8.4.13 NEW PRODUCT INTRODUCTION SERVICES
8.4.14 MANUFACTURING SERVICES
8.4.15 ENVIRONMENTAL AND REGULATORY SERVICES
8.4.16 MEDICAL MANAGEMENT SYSTEMS CERTIFICATION & AUDITING
8.4.17 CLINICAL RESEARCH SERVICES
8.4.18 CONSULTATIVE SERVICES
8.4.19 OTHER SERVICES
8.5 OTHERS
9 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION
9.1 NORTH AMERICA
9.1.1 U.S.
9.1.2 CANADA
9.1.3 MEXICO
10 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: COMPANY LANDSCAPE
10.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
11 SWOT ANALYSIS
12 COMPANY PROFILE
12.1 F.HOFFMANN-LA ROCHE LTD
12.1.1 COMPANY SNAPSHOT
12.1.2 REVENUE ANALYSIS
12.1.3 COMPANY SHARE ANALYSIS
12.1.4 SERVICE PORTFOLIO
12.1.5 RECENT DEVELOPMENT
12.2 DANAHER
12.2.1 COMPANY SNAPSHOT
12.2.2 REVENUE ANALYSIS
12.2.3 COMPANY SHARE ANALYSIS
12.2.4 PRODUCT PORTFOLIO
12.2.5 RECENT DEVELOPMENT
12.3 BIOMERIEUX
12.3.1 COMPANY SNAPSHOT
12.3.2 REVENUE ANALYSIS
12.3.3 COMPANY SHARE ANALYSIS
12.3.4 SERVICE PORTFOLIO
12.3.5 RECENT DEVELOPMENT
12.4 QIAGEN
12.4.1 COMPANY SNAPSHOT
12.4.2 REVENUE ANALYSIS
12.4.3 COMPANY SHARE ANALYSIS
12.4.4 SERVICE PORTFOLIO
12.4.5 RECENT DEVELOPMENT
12.5 THERMO FISHER SCIENTIFIC
12.5.1 COMPANY SNAPSHOT
12.5.2 REVENUE ANALYSIS
12.5.3 COMPANY SHARE ANALYSIS
12.5.4 SERVICE PORTFOLIO
12.5.5 RECENT DEVELOPMENT
12.6 ABBOTT
12.6.1 COMPANY SNAPSHOT
12.6.2 REVENUE ANALYSIS
12.6.3 SERVICE PORTFOLIO
12.6.4 RECENT DEVELOPMENT
12.7 BIO-RAD LABORATORIES, INC
12.7.1 COMPANY SNAPSHOT
12.7.2 REVENUE ANALYSIS
12.7.3 SERVICE PORTFOLIO
12.7.4 RECENT DEVELOPMENTS
12.8 DIASORIN
12.8.1 COMPANY SNAPSHOT
12.8.2 REVENUE ANALYSIS
12.8.3 SERVICE PORTFOLIO
12.8.4 RECENT DEVELOPMENT
12.9 HOLOGIC, INC
12.9.1 COMPANY SNAPSHOT
12.9.2 REVENUE ANALYSIS
12.9.3 SERVICE PORTFOLIO
12.9.4 RECENT DEVELOPMENT
13 QUESTIONNAIRE
14 RELATED REPORTS
表格列表
TABLE 1 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 1 NORTH AMERICA INSTRUMENT REPAIR SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 2 NORTH AMERICA INSTRUMENT REPAIR SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 3 NORTH AMERICA MAINTENANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 4 NORTH AMERICA TRAINING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 5 NORTH AMERICA TRAINING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 NORTH AMERICA CALIBRATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 7 NORTH AMERICA COMPLIANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 8 NORTH AMERICA COMPLIANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 NORTH AMERICA TURNKEY SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 10 NORTH AMERICA SCALABLE AUTOMATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 11 NORTH AMERICA SCALABLE AUTOMATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 12 NORTH AMERICA HARDWARE CUSTOMIZATION IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 13 NORTH AMERICA INSTRUMENT RELOCATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 14 NORTH AMERICA INSTRUMENT RELOCATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 15 NORTH AMERICA PERFORMANCE ASSURANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 16 NORTH AMERICA DESIGN AND DEVELOPMENT SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 17 NORTH AMERICA SUPPLY CHAIN SOLUTIONS SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 18 NORTH AMERICA NEW PRODUCT INTRODUCTION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 19 NORTH AMERICA MANUFACTURING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 20 NORTH AMERICA ENVIRONMENTAL AND REGULATORY SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 21 NORTH AMERICA MEDICAL MANAGEMENT SYSTEMS AND AUDITING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 22 NORTH AMERICA CLINICAL RESEARCH SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 23 NORTH AMERICA CONSULTATIVE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 24 NORTH AMERICA OTHER SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 25 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY TECHNOLOGY, 2021-2030 (USD THOUSAND)
TABLE 26 NORTH AMERICA REAL TIME PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 27 NORTH AMERICA REAL TIME PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 28 NORTH AMERICA PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 29 NORTH AMERICA PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 30 NORTH AMERICA NEXT GENERATION SEQUENCING IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 31 NORTH AMERICA NEXT GENERATION SEQUENCING IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 32 NORTH AMERICA OTHER TECHNOLOGIES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 33 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 34 NORTH AMERICA HOSPITALS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 35 NORTH AMERICA HOSPITALS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 36 NORTH AMERICA DIAGNOSTIC CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 37 NORTH AMERICA DIAGNOSTIC CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 38 NORTH AMERICA CLINICAL LABORATORIES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 39 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 40 NORTH AMERICA ACADEMIC AND RESEARCH INSTITUTES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 41 NORTH AMERICA ACADEMIC AND RESEARCH INSTITUTES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 42 NORTH AMERICA OTHERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 43 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 44 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 45 NORTH AMERICA INSTRUMENT REPAIR SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 46 NORTH AMERICA TRAINING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 47 NORTH AMERICA COMPLIANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 48 NORTH AMERICA SCALABLE AUTOMATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 49 NORTH AMERICA INSTRUMENT RELOCATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 50 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY TECHNOLOGY, 2021-2030 (USD THOUSAND)
TABLE 51 NORTH AMERICA REAL TIME PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 52 NORTH AMERICA PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 53 NORTH AMERICA NEXT GENERATION SEQUENCING IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 54 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 55 NORTH AMERICA HOSPITALS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE 2021-2030 (USD THOUSAND)
TABLE 56 NORTH AMERICA DIAGNOSTIC CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 57 NORTH AMERICA CLINICAL LABORATORIES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 58 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 59 NORTH AMERICA ACADEMIC & RESEARCH INSTITUTES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 60 U.S. MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 61 U.S. INSTRUMENT REPAIR SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 62 U.S. TRAINING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 63 U.S. COMPLIANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 64 U.S. SCALABLE AUTOMATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 65 U.S. INSTRUMENT RELOCATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 66 U.S. MOLECULAR DIAGNOSTICS SERVICES MARKET, BY TECHNOLOGY, 2021-2030 (USD THOUSAND)
TABLE 67 U.S. REAL TIME PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 68 U.S. PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 69 U.S. NEXT GENERATION SEQUENCING IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 70 U.S. MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 71 U.S. HOSPITALS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE 2021-2030 (USD THOUSAND)
TABLE 72 U.S. DIAGNOSTIC CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 73 U.S. CLINICAL LABORATORIES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 74 U.S. AMBULATORY SURGICAL CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 75 U.S. ACADEMIC & RESEARCH INSTITUTES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 76 CANADA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 77 CANADA INSTRUMENT REPAIR SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 78 CANADA TRAINING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 79 CANADA COMPLIANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 80 CANADA SCALABLE AUTOMATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 81 CANADA INSTRUMENT RELOCATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 82 CANADA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY TECHNOLOGY, 2021-2030 (USD THOUSAND)
TABLE 83 CANADA REAL TIME PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 84 CANADA PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 85 CANADA NEXT GENERATION SEQUENCING IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 86 CANADA MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 87 CANADA HOSPITALS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE 2021-2030 (USD THOUSAND)
TABLE 88 CANADA DIAGNOSTIC CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 89 CANADA CLINICAL LABORATORIES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 90 CANADA AMBULATORY SURGICAL CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 91 CANADA ACADEMIC & RESEARCH INSTITUTES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 92 MEXICO MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 93 MEXICO INSTRUMENT REPAIR SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 94 MEXICO TRAINING SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 95 MEXICO COMPLIANCE SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 96 MEXICO SCALABLE AUTOMATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 97 MEXICO INSTRUMENT RELOCATION SERVICES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 98 MEXICO MOLECULAR DIAGNOSTICS SERVICES MARKET, BY TECHNOLOGY, 2021-2030 (USD THOUSAND)
TABLE 99 MEXICO REAL TIME PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 100 MEXICO PCR IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 101 MEXICO NEXT GENERATION SEQUENCING IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 102 MEXICO MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 103 MEXICO HOSPITALS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE 2021-2030 (USD THOUSAND)
TABLE 104 MEXICO DIAGNOSTIC CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 105 MEXICO CLINICAL LABORATORIES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 106 MEXICO AMBULATORY SURGICAL CENTERS IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
TABLE 107 MEXICO ACADEMIC & RESEARCH INSTITUTES IN MOLECULAR DIAGNOSTICS SERVICES MARKET, BY SERVICE TYPE, 2021-2030 (USD THOUSAND)
图片列表
FIGURE 1 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE OF CHRONIC AND INFECTIOUS DISEASES IS EXPECTED TO DRIVE THE NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 SERVICE TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET
FIGURE 14 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET : BY SERVICE TYPE, 2022
FIGURE 15 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET : BY SERVICE TYPE, 2023-2030 (USD THOUSAND)
FIGURE 16 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET : BY SERVICE TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET : BY SERVICE TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY TECHNOLOGY, 2022
FIGURE 19 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY TECHNOLOGY, 2023-2030 (USD THOUSAND)
FIGURE 20 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 22 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY END USER, 2022
FIGURE 23 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY END USER, 2023-2030 (USD THOUSAND)
FIGURE 24 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY END USER, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET : BY END USER, LIFELINE CURVE
FIGURE 26 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: SNAPSHOT (2022)
FIGURE 27 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY COUNTRY (2022)
FIGURE 28 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY COUNTRY (2023 & 2030)
FIGURE 29 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY COUNTRY (2022 & 2030)
FIGURE 30 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: BY SERVICE TYPE (2023-2030)
FIGURE 31 NORTH AMERICA MOLECULAR DIAGNOSTICS SERVICES MARKET: COMPANY SHARE 2022 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。













