北美抗病毒药物市场,按适应症(流感、人类免疫缺陷病毒 (HIV)、丙型肝炎病毒 (HCV)、呼吸道合胞病毒、单纯疱疹病毒、人类巨细胞病毒 (HCMV)、水痘-带状疱疹病毒 (VZV)、乙型肝炎病毒 (HBV)、冠状病毒感染等)、患者类型(儿童、成人和老年人)、产品(口服、外用和肠外用药)、药物类型(仿制药和品牌药)、最终用户(医院、诊所、家庭医疗保健、专科中心、门诊中心等)、分销渠道(医院药房、网上药房和零售药房)划分 - 行业趋势和预测到 2030 年。
北美抗病毒药物市场分析与洞察
北美对病毒感染的认识不断提高,增强了市场需求。为获得更好的医疗服务而增加的医疗支出也促进了市场的增长。在这一关键时期,主要市场参与者专注于各种服务的推出和批准。此外,药物开发技术的进步也促进了对抗病毒药物的需求增加。
由于市场参与者的增加和先进服务的可用性,北美抗病毒药物市场预计在预测年将增长。与此同时,制造商正致力于开发活动,以在市场上推出新服务。药物开发领域的不断发展进一步推动了市场的增长。然而,缺乏标准化协议和缺乏熟练的专业人员等困难可能会阻碍预测期内北美抗病毒药物市场的增长。
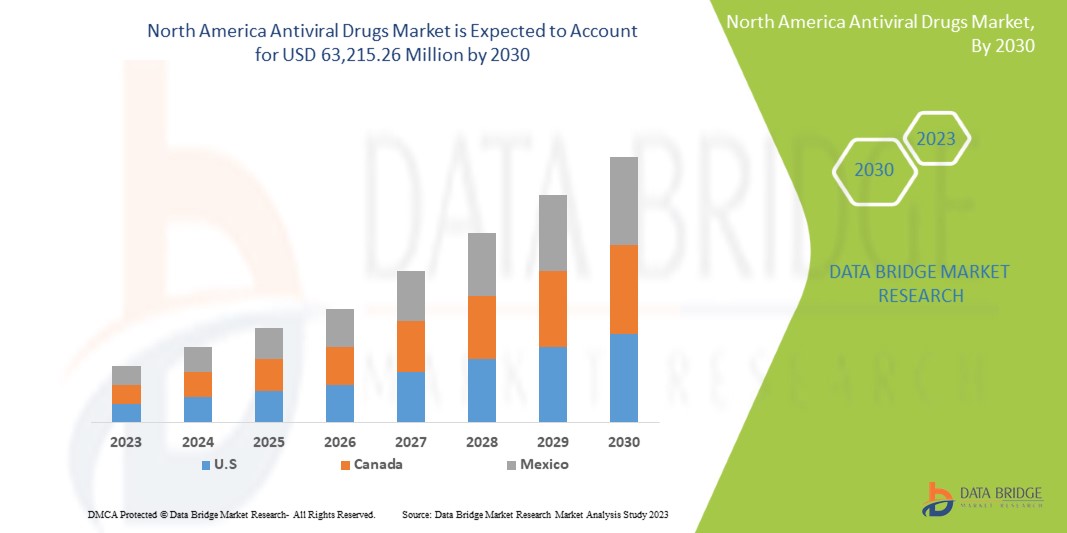
医疗保健在进步和药物开发方面的支出增加预计将为市场带来机遇。然而,替代疗法的日益普及可能会对市场增长构成挑战。Data Bridge Market Research 分析称,预计到 2030 年,北美抗病毒药物市场价值将达到 632.1526 亿美元,预测期内复合年增长率为 5.5%。
|
报告指标 |
细节 |
|
预测期 |
2023 至 2030 年 |
|
基准年 |
2022 |
|
历史岁月 |
2021 (可定制为 2015-2020) |
|
定量单位 |
收入(百万美元)、销量(单位)和定价(美元) |
|
涵盖的领域 |
适应症(流感、人类免疫缺陷病毒 (HIV)、丙型肝炎病毒 (HCV)、呼吸道合胞病毒、单纯疱疹病毒、人类巨细胞病毒 (HCMV)、水痘-带状疱疹病毒 (VZV)、乙型肝炎病毒 (HBV)、冠状病毒感染等)、患者类型(儿童、成人和老年人)、产品(口服、外用和肠外用药)、药品类型(仿制药和品牌药)、最终用户(医院、诊所、家庭医疗保健、专科中心、门诊中心等)、分销渠道(医院药房、网上药房和零售药房) |
|
覆盖国家 |
美国、加拿大和墨西哥 |
|
涵盖的市场参与者 |
Gilead Sciences, Inc.、F. Hoffmann-La Roche Ltd、GLAXOSMITHKLINE PLC、Abbvie、Merck & Co., Inc.、Johnson & Johnson Services, Inc.、Bristol-Myers Squibb Company、Cipla Inc.、Aurobindo Pharma、Dr. Reddy's Laboratories Ltd.、Zydus Pharmaceuticals, Inc.、Mylan Pharmaceuticals ULC、Teva Pharmaceuticals USA, Inc.、EMERGENT、Sun Pharmaceutical Industries Ltd.、Avet Pharmaceuticals Inc.、Pfizer Inc.、SIGA Technologies、NAVINTA LLC.、Macleods Pharmaceuticals Ltd.、BioCryst Pharmaceuticals, Inc 和 Hetero. 等等 |
北美抗病毒药物市场定义
抗病毒药物是通过抑制宿主细胞内病毒的复制来治疗病毒感染的药物。这些药物针对特定病毒或病毒类型,通过阻止病毒进入宿主细胞或阻断病毒复制所需的关键酶或蛋白质起作用。与用于治疗细菌感染的抗生素不同,抗病毒药物通常效果较差,因为病毒的结构要简单得多,并且依赖宿主细胞进行复制。然而,它们仍然可用于治疗某些病毒感染,例如流感、疱疹和艾滋病毒。
北美抗病毒药物市场动态
本节旨在了解市场驱动因素、机遇、限制因素和挑战。下文将详细讨论所有这些内容:
驱动程序
- 病毒感染患病率上升
过去几十年来,病毒感染在世界各地稳步增加。病毒进入人体,利用其细胞进行复制并传播,从而引起病毒感染。病毒感染可导致各种症状,从轻微到严重,在某些情况下甚至危及生命。全球化是病毒感染增加的主要原因之一。人们跨越国界旅行和交流,使世界比以往任何时候都更加紧密地联系在一起。由于这种联系的增强,病毒从一个地区传播到另一个地区的速度加快了。
因此,病毒感染的流行率上升是一个复杂的问题,有许多因素。全球化、人口密度、气候变化和抗生素耐药性都会影响病毒的传播。因此,预计它将推动市场增长。
- 新型抗病毒药物研发取得进展
抗病毒治疗是针对病毒感染患者的处方药。新型抗病毒药物的研发在历史上取得了巨大进步。这些进展降低了疾病负担,改善了病毒感染的治疗,挽救了生命。新型抗病毒药物领域取得了很大进展。
因此,新型抗病毒药物开发的进步改善了病毒感染的治疗,减轻了疾病负担,并有望推动市场增长。
克制
- 抗病毒药物成本高昂
抗病毒药物的高昂价格可能对患者和医疗保健系统产生重大影响。买不起这些药物的患者可能会得不到治疗或依赖劣质治疗,导致健康状况恶化。此外,抗病毒药物的高昂价格可能会给医疗保健预算造成压力,尤其是在资源有限的国家。
因此,抗病毒药物的高成本预计会抑制北美抗病毒药物市场的发展。
机会
-
新兴药物输送系统
抗病毒药物研究的重点是开发新型药物输送机制。与传统药物给药技术相比,新型输送系统具有多项优势,例如生物利用度更高、药物输送量身定制、副作用更少。
因此,开发新型药物输送系统是抗病毒药物研究的一个重要领域。纳米颗粒药物输送系统、水凝胶、树枝状聚合物、微针和细胞穿透肽是一些有前途的药物输送系统,已被用于抗病毒药物。这些输送系统比传统的药物输送方法具有多种优势,有可能提高抗病毒药物的疗效和安全性,预计将为市场增长创造机会。
挑战
- 抗病毒药物专利到期
专利到期会导致原始开发者或专利持有者失去生产和销售特定药物的独家权利。抗病毒药物的专利到期可能会对制药业产生重大影响,因为它可能会引发仿制药生产商的竞争。
抗病毒药物的专利到期后,其发明者将拥有制造和销售该药物的独家权利。药物专利到期后,其他生产商可以制造和销售仿制药。这可能会导致竞争加剧和消费者价格下降。艾滋病毒、乙肝和丙肝、疱疹、流感和其他病毒性疾病都可以用抗病毒药物治疗。抗病毒药物的专利到期时间因药物和国家而异。药物专利通常从申请之日起授予 20 年。专利到期后,其他生产商可以自由制造和销售仿制药。由于生产商不必在营销、研发和临床研究上投入太多资金,仿制药通常比名牌药物更实惠。
因此,抗病毒药物的专利到期可能对这些重要药物的可用性、可负担性和可及性产生重大影响,并预计会对市场增长构成挑战。
最新动态
- 2023 年 1 月,默克公司(简称 MSD)宣布通过一家子公司成功完成对 Imago Biosciences, Inc.(纳斯达克股票代码:IMGO)所有流通普通股的现金要约收购,收购价格为每股 36.00 美元,不计利息,但需扣除任何必要的预扣税。此次收购将有助于增加收入。
- 2021 年 4 月,Zydus Pharmaceuticals, Inc. 宣布已获得印度药品管理总局 (DCGI) 的限制性紧急使用批准,可使用抗病毒药物 Virafin 治疗中度 COVID-19 感染。这将有助于该公司提高其在北美的影响力和在全球其他地区的声誉。
北美抗病毒药物市场范围
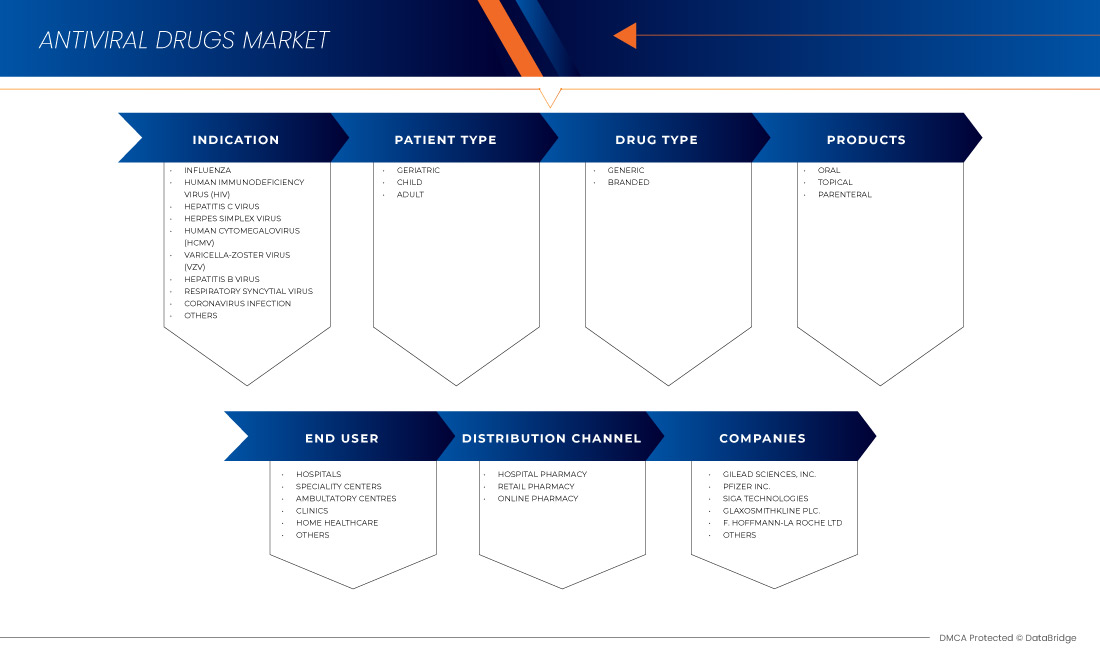
北美抗病毒药物市场根据适应症、患者类型、产品、药物类型、最终用户和分销渠道分为六个显著的细分市场。细分市场之间的增长有助于您分析利基增长领域和进入市场的策略,并确定您的核心应用领域和目标市场的差异。
指征
- 流感
- 人类免疫缺陷病毒(HIV)
- 丙型肝炎病毒
- 单纯疱疹病毒
- 人类巨细胞病毒 (HCMV)
- 水痘-带状疱疹病毒 (VZV)
- 乙型肝炎病毒
- 呼吸道合胞病毒
- 冠状病毒感染
- 其他的
根据适应症,北美抗病毒药物市场分为流感、人类免疫缺陷病毒 (HIV)、丙型肝炎病毒 (HCV)、呼吸道合胞病毒、单纯疱疹病毒、人类巨细胞病毒 (HCMV)、水痘带状疱疹病毒 (VZV)、乙型肝炎病毒 (HBV)、冠状病毒感染等。
患者类型
- 孩子
- 成人
- 老年
根据患者类型,北美抗病毒药物市场分为儿童、成人和老年人。
产品
- Oral
- Topical
- Parenteral
On the basis of products, the North America antiviral drugs market is segmented into oral, topical, and parenteral.
Drug Type
- Generic
- Branded
On the basis of drug type, the North America antiviral drugs market is segmented into generic and branded.
End User
- Hospital
- Clinics
- Home Healthcare
- Speciality Centers
- Ambulatory Centers
- Others
On the basis of end user, the North America antiviral drugs market is segmented into hospitals, clinics, home healthcare, specialty centers, ambulatory centers, and others.
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
On the basis of distribution channel, the North America antiviral drugs market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy.
North America Antiviral Drugs Market Regional Analysis/Insights
The North America antiviral drugs market is categorized into six notable segments based on indication, patient type, products, drug type, end user, and distribution channel.
The countries covered in this market report U.S., Canada, and Mexico.
In 2023, U.S., dominates the North America region due to the strong presence of key players and due to the increasing demand from emerging markets and expansion
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Antiviral Drugs Market Share Analysis
North America antiviral drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product approvals, product width and breath, application dominance, and product type lifeline curve. The above data points provided are only related to the company’s focus on the North America antiviral drugs market.
北美抗病毒药物市场的一些主要参与者包括 Gilead Sciences, Inc.、F. Hoffmann-La Roche Ltd、GLAXOSMITHKLINE PLC、Abbvie、Merck & Co., Inc.、Johnson & Johnson Services, Inc.、Bristol-Myers Squibb Company、Cipla Inc.、Aurobindo Pharma、Dr. Reddy's Laboratories Ltd.、Zydus Pharmaceuticals, Inc.、Mylan Pharmaceuticals ULC、Teva Pharmaceuticals USA, Inc.、EMERGENT、Sun Pharmaceutical Industries Ltd.、Avet Pharmaceuticals Inc.、Pfizer Inc.、SIGA Technologies、NAVINTA LLC.、Macleods Pharmaceuticals Ltd.、BioCryst Pharmaceuticals, Inc 和 Hetero 等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR NORTH AMERICA ANTIVIRAL DRUGS MARKET
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING PREVALENCE OF VIRAL INFECTIONS
8.1.2 ADVANCEMENTS IN NEW ANTIVIRAL DRUG DEVELOPMENT
8.1.3 GROWING DEMAND FOR COMBINATION THERAPIES
8.1.4 INCREASING GOVERNMENT FUNDING AND R&D ACTIVITIES
8.2 RESTRAINS
8.2.1 HIGH COST OF ANTIVIRAL DRUGS
8.2.2 EMERGENCE OF DRUG-RESISTANT STRAINS OF VIRUSES
8.3 OPPORTUNITIES
8.3.1 INCREASING COLLABORATION AND PARTNERSHIP AMONG KEY PLAYERS
8.3.2 RISING NOVEL DRUG DELIVERY SYSTEMS
8.3.3 DEVELOPMENT OF PERSONALIZED MEDICINES
8.4 CHALLENGES
8.4.1 PATENT EXPIRATION OF ANTIVIRAL DRUGS
8.4.2 DEMAND FOR ALTERNATIVE MEDICINES
9 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION
9.1 OVERVIEW
9.2 INFLUENZA
9.2.1 NEURAMINIDASE INHIBITORS
9.2.1.1 OSELTAMIVIR
9.2.1.2 ZANAMIVIR
9.2.1.3 PERAMIVIR
9.2.1.4 LANINAMIVIR
9.2.2 M2 INHIBITORS
9.2.2.1 RIMANTADINE
9.2.2.2 OTHERS
9.2.3 RNA POLYMERASE INHIBITORS
9.2.3.1 FAVIPIRAVIR
9.2.3.2 BALOXAVIR MARBOXIL
9.3 HUMAN IMMUNODEFICIENCY VIRUS (HIV)
9.3.1 REVERSE TRANSCRIPTASE INHIBITORS
9.3.1.1 NUCLEOSIDE (NRTIS)
9.3.1.1.1 LAMIVUDINE
9.3.1.1.2 ABACAVIR
9.3.1.1.3 DIDANOSINE
9.3.1.1.4 OTHERS
9.3.1.2 NONNUCLEOSIDE (NNRTIS)
9.3.1.2.1 EFAVIRENZ
9.3.1.2.2 NEVIRAPINE
9.3.1.2.3 DELAVIRDINE
9.3.1.2.4 OTHERS
9.3.1.3 INTEGRASE
9.3.1.3.1 DOLUTEGRAVIR
9.3.1.3.2 ELVITEGRAVIR
9.3.1.3.3 RALTEGRAVIR
9.3.1.3.4 BICTEGRAVIR
9.3.1.4 NUCLEOTIDE
9.3.1.4.1 TENOFOVIR
9.3.1.4.2 OTHERS
9.3.1.5 INTERFERONS
9.3.1.5.1 ALPHA
9.3.1.5.2 BETA
9.3.1.5.3 GAMMA
9.3.1.6 GP41
9.3.1.6.1 ENFUVIRTIDE
9.3.1.6.2 OTHERS
9.3.2 PROTEASE
9.3.2.1 ATAZANAVIR
9.3.2.2 DARUNAVIR
9.3.2.3 LOPINAVIR
9.3.2.4 RITONAVIR
9.3.2.5 SAQUINAVIR
9.3.2.6 INDINAVIR
9.3.2.7 NELFINAVIR
9.3.2.8 TIPRANAVIR
9.3.2.9 AMPRENAVIR
9.4 HEPATITIS C VIRUS
9.4.1 NS5B POLYMERASE
9.4.1.1 SOFOSBUVIR
9.4.1.2 DASABUVIR
9.4.2 NS3/4A PROTEASE
9.4.2.1 DANOPREVIR
9.4.2.2 GLECAPREVIR
9.4.2.3 GRAZOPREVIR
9.4.2.4 PARITAPREVIR
9.4.2.5 SIMEPREVIR
9.4.3 NS5A PHOSPHOPROTEIN
9.4.3.1 LEDIPASVIR
9.4.3.2 VELPATASVIR
9.4.3.3 OMBITASVIR
9.4.3.4 ELBASVIR
9.4.3.5 DACLATASVIR
9.4.3.6 PIBRENTASVIR
9.4.4 NEURAMINIDASE
9.4.4.1 OSELTAMIVIR
9.4.4.2 ZANAMIVIR
9.4.4.3 PERAMIVIR
9.4.4.4 LANINAMIVIR
9.4.5 RNA POLYMERASE
9.4.5.1 BALOXAVIR MARBOXIL
9.4.5.2 FAVIPIRAVIR
9.4.6 MATRIX PROTEIN 2
9.4.6.1 RIMATIDINE
9.4.6.2 FAVIPIRAVIR
9.5 HERPES SIMPLEX VIRUS
9.5.1 DNA POLYMERASE UL30
9.5.1.1 ACICLOVIR
9.5.1.2 FAMCICLOVIR
9.5.1.3 VALACICLOVIR
9.5.1.4 PENCICLOVIR TRIFLURIDINE
9.5.1.5 BRIVUDINE
9.5.1.6 FOSCARNET
9.5.1.7 IDOXURIDINE
9.5.2 ENVELOPE PROTEINS
9.5.2.1 DOCOSANOL
9.5.2.2 OTHERS
9.6 HUMAN CYTOMEGALOVIRUS (HCMV)
9.6.1 GANCICLOVIR
9.6.2 VALGANCICLOVIR
9.6.3 CIDOFOVIR
9.6.4 FOSCARNET
9.6.5 FOMIVIRSEN
9.7 VARICELLA-ZOSTER VIRUS (VZV)
9.7.1 VALACICLOVIR
9.7.2 FAMCICLOVIR
9.7.3 ACICLOVIR
9.7.4 VIDARABINE
9.7.5 BRIVUDINE
9.8 HEPATITIS B VIRUS
9.8.1 ENTECAVIR
9.8.2 TENOFOVIR
9.8.3 TELBIVUDINE
9.8.4 TENOFOVIR ALAFENAMIDE
9.8.5 OTHERS
9.9 RESPIRATORY SYNCYTIAL VIRUS
9.9.1 RNA POLYMERASE
9.9.1.1 RIBAVIRIN
9.9.1.2 OTHERS
9.9.2 FUSION GLYCOPROTEIN
9.9.2.1 PALIVIZUMAB
9.9.2.2 OTHERS
9.1 CORONAVIRUS INFECTION
9.11 OTHERS
10 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 GERIATRIC
10.2.1 MALE
10.2.2 FEMALE
10.3 CHILD
10.3.1 MALE
10.3.2 FEMALE
10.4 ADULT
10.4.1 MALE
10.4.2 FEMALE
11 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS
11.1 OVERVIEW
11.2 ORAL
11.2.1 SOLID
11.2.1.1 TABLETS
11.2.1.2 CAPSULES
11.2.1.3 OTHERS
11.2.2 SEMISOLID
11.2.2.1 GELS
11.2.2.2 EMULSIONS
11.2.2.3 ELIXIRS
11.2.2.4 OTHERS
11.2.3 LIQUID
11.2.3.1 SOLUTIONS
11.2.3.2 SYRUPS
11.2.3.3 OTHERS
11.3 TOPICAL
11.3.1 SEMI-SOLID
11.3.1.1 CREAM
11.3.1.2 OINTMENT
11.3.1.3 GELS
11.3.1.4 OTHERS
11.3.2 LIQUID
11.3.2.1 SOLUTIONS
11.3.2.2 SUSPENSIONS
11.3.3 SOLID
11.3.3.1 POWDERS
11.3.3.2 SUPPOSITORIES
11.3.3.3 ENEMA
11.3.3.4 OTHERS
11.4 PARENTERAL
11.4.1 CONVENTIONAL DRUG DELIVERY FORMUALTIONS
11.4.1.1 SOLUTIONS
11.4.1.2 RECONSTITUTED/LYOPHILIZED
11.4.1.3 SUSPENSIONS
11.4.1.4 EMULSIONS
11.4.1.5 OTHERS
11.4.2 NOVEL DRUG DELIVERY FORMULATIONS
11.4.3 COLLOIDAL DISPERSIONS
11.4.4 LONG ACTING INJECTION FORMULATION
12 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERIC
12.3 BRANDED
13 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CENTERS
13.4 AMBULATORY CENTRES
13.5 CLINICS
13.6 HOME HEALTHCARE
13.7 OTHERS
14 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 GILEAD SCIENCES, INC. (2022)
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 PFIZER INC. (2022)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 SIGA TECHNOLOGIES (2022)
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 GLAXOSMITHKLINE PLC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENT
18.5 F. HOFFMANN-LA ROCHE LTD. (2022)
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBVIE INC.
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 AUROBINDO PHARMA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 AVET PHARMACEUTICALS INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BRISTOLL MYERS SQUIBB (2022)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BIOCRYST PHARMACEUTICALS, INC. (2022)
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 CIPLA INC. (2022)
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENT
18.12 DR. REDDY’S LABORATORIES LTD. (2022)
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 EMERGENT (2022)
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 HETERO.
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENT
18.15 JOHNSON & JOHNSON PRIVATE LIMITED (2022)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MACLEODS PHARMACEUTICALS LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MERCK & CO., INC, (2022)
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENT
18.18 MYLAN N.V (SUBSIDIARY OF VIATRIS) (2022)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 NAVINTA LLC.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 SUN PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 TEVA PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENT
18.22 ZYDUS PHARMACEUTICALS, INC. (2022)
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
表格列表
TABLE 1 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021- 2030 (USD MILLION)
TABLE 2 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA HUMAN CYTOMEGALOVIRUS (HCMV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA HEPATITIS B VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA RESPIRATORY SYNCYTIAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA CORONAVIRUS INFECTION IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021- 2030 (USD MILLION)
TABLE 42 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCT, 2021- 2030 (USD MILLION)
TABLE 49 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021- 2030 (USD MILLION)
TABLE 64 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021- 2030 (USD MILLION)
TABLE 65 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021- 2030 (USD MILLION)
TABLE 66 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 83 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 84 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 86 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 87 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 98 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 99 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 100 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 101 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 102 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 103 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 104 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 105 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 106 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 107 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 108 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 109 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 110 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 111 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 112 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 113 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 114 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 115 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 116 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 117 U.S. ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 118 U.S. INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.S. NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 120 U.S. M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 121 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 122 U.S. HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 123 U.S. REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 124 U.S. NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 125 U.S. NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 126 U.S. INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 127 U.S. NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 128 U.S. INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 129 U.S. GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 130 U.S. PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 131 U.S. HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 132 U.S. NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 133 U.S. NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 134 U.S. NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 135 U.S. NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 136 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 137 U.S. MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 138 U.S. HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 139 U.S. DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 140 U.S. ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 141 U.S. HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 142 U.S. VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 143 U.S. DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 144 U.S. RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 145 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 146 U.S. FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 147 U.S. ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 148 U.S. GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 149 U.S. CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 150 U.S. ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 151 U.S. ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 152 U.S. ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 153 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 154 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 155 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 156 U.S. TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 157 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 158 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 159 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 160 U.S. PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 161 U.S.CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 162 U.S. NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 163 U.S. ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 164 U.S. ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 U.S. ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 CANADA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 167 CANADA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 168 CANADA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 169 CANADA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 170 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 171 CANADA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 172 CANADA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 173 CANADA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 174 CANADA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 175 CANADA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 176 CANADA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 177 CANADA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 178 CANADA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 179 CANADA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 180 CANADA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 181 CANADA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 182 CANADA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 183 CANADA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 184 CANADA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 185 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 186 CANADA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 187 CANADA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 188 CANADA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 189 CANADA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 190 CANADA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 191 CANADA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 192 CANADA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 193 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 194 CANADA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 195 CANADA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 196 CANADA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 197 CANADA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 198 CANADA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 199 CANADA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 200 CANADA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 201 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 202 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 203 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 204 CANADA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 205 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 206 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 207 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 208 CANADA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 209 CANADA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 210 CANADA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 211 CANADA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 212 CANADA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 CANADA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 MEXICO ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 215 MEXICO INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 216 MEXICO NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 217 MEXICO M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 218 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 219 MEXICO HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 220 MEXICO REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 221 MEXICO NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 222 MEXICO NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 223 MEXICO INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 224 MEXICO NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 225 MEXICO INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 226 MEXICO GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 227 MEXICO PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 228 MEXICO HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 229 MEXICO NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 230 MEXICO NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 231 MEXICO NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 232 MEXICO NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 233 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 234 MEXICO MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 235 MEXICO HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 236 MEXICO DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 237 MEXICO ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 238 MEXICO HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 239 MEXICO VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 240 MEXICO DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 241 MEXICO RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 242 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 243 MEXICO FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 244 MEXICO ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 245 MEXICO GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 246 MEXICO CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 247 MEXICO ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 248 MEXICO ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 249 MEXICO ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 250 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 251 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 252 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 253 MEXICO TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 254 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 255 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 256 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 257 MEXICO PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 258 MEXICO CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 259 MEXICO NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 260 MEXICO ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 261 MEXICO ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 262 MEXICO ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
图片列表
FIGURE 1 NORTH AMERICA ANTIVIRAL DRUGS: SEGMENTATION
FIGURE 2 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA ANTIVIRAL DRUGS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ANTIVIRAL DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ANTIVIRAL DRUGS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA ANTIVIRAL DRUGS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE OF VIRAL INFECTIONS IS EXPECTED TO DRIVE THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INFLUENZA SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
FIGURE 14 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2022
FIGURE 15 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , CAGR (2023-2030)
FIGURE 17 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , LIFELINE CURVE
FIGURE 18 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2022
FIGURE 19 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2022
FIGURE 23 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2022
FIGURE 27 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2022
FIGURE 31 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 35 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SNAPSHOT (2022)
FIGURE 39 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022)
FIGURE 40 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION (2023-2030)
FIGURE 43 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY SHARE 2022 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。