Global Nucleic Acid Amplification Market
市场规模(十亿美元)
CAGR :
% 
 USD
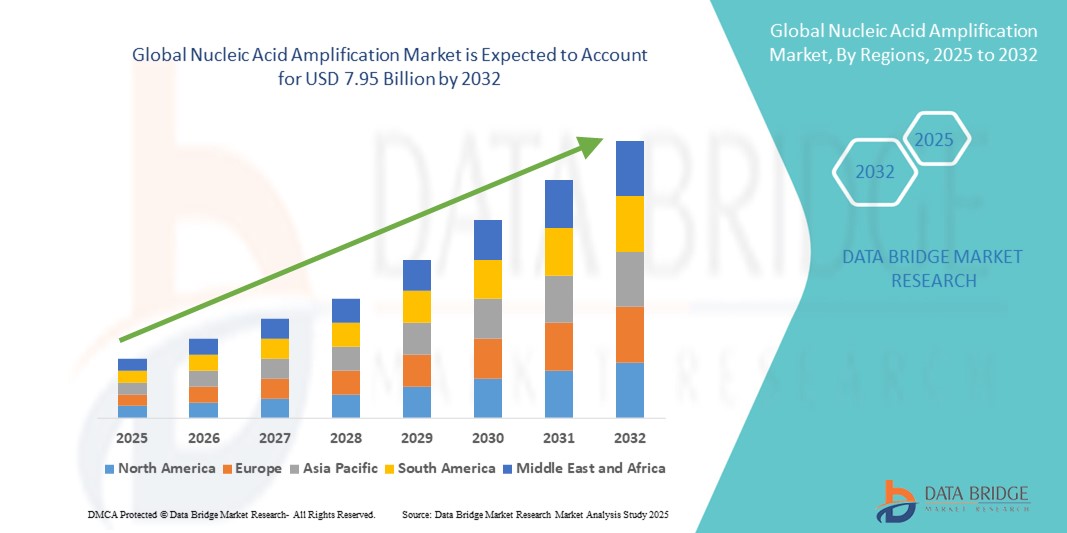
3.90 Billion
USD
7.95 Billion
2024
2032
USD
3.90 Billion
USD
7.95 Billion
2024
2032
| 2025 –2032 | |
| USD 3.90 Billion | |
| USD 7.95 Billion | |
|
|
|
|
全球核酸擴增市場細分,按應用(傳染病、癌症、個人化醫療和遺傳)、按技術(標靶擴增、探針擴增和訊號擴增)——行業趨勢和預測(至 2032 年)
核酸擴增市場規模
- 2024 年全球核酸擴增市場規模為39 億美元 ,預計 到 2032 年將達到 79.5 億美元,預測期內 複合年增長率為 9.30%。
- 市場成長主要受醫療保健、研究和法醫應用對快速、靈敏、準確的分子診斷技術的需求不斷增長的推動
- 此外,擴增技術的進步、傳染病發病率的上升以及個人化醫療應用的不斷擴展,正在推動全球範圍內的採用。這些因素共同加速了核酸擴增方法的整合,從而顯著推動了市場的成長。
核酸擴增市場分析
- 核酸擴增技術可以快速、精確地檢測遺傳物質,成為分子診斷、傳染病檢測以及臨床和實驗室環境中遺傳研究的重要工具
- 不斷增長的需求主要是由於傳染病的日益流行、PCR和等溫擴增等擴增技術的進步以及個人化醫療和癌症檢測中分子診斷的日益普及
- 北美在核酸擴增市場佔據主導地位,2024 年的收入份額最大,為 39.4%,這得益於先進的醫療基礎設施、高額的研發投入以及診斷檢測和平台領域關鍵參與者的創新
- 由於醫療保健管道的擴大、傳染病負擔的增加以及政府推動分子診斷的舉措不斷增加,預計亞太地區將成為預測期內核酸擴增市場成長最快的地區
- 傳染病領域在核酸擴增市場佔據主導地位,2024 年的市佔率為 52.5%,這主要得益於臨床環境中對快速病原體檢測和疫情管理的迫切需求
報告範圍和核酸擴增市場細分
|
屬性 |
核酸擴增關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
北美洲
歐洲
亞太
中東和非洲
南美洲
|
|
主要市場參與者 |
|
|
市場機會 |
|
|
加值資料資訊集 |
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深入的專家分析、定價分析、品牌份額分析、消費者調查、人口統計分析、供應鏈分析、價值鏈分析、原材料/消耗品概述、供應商選擇標準、PESTLE 分析、波特分析和監管框架。 |
核酸擴增市場趨勢
快速和即時檢測技術的進步
- 全球核酸擴增市場的一個重要且正在加速發展的趨勢是開發和採用快速、便攜式即時診斷設備 (POC),以便在傳統實驗室環境之外提供及時的結果
- 例如,Cepheid 的 GeneXpert 和雅培的 ID NOW 等平台能夠快速檢測 COVID-19 和流感等傳染源,透過更快的臨床決策改善患者的治療效果
- LAMP(環介導等溫擴增)等溫擴增技術的創新允許在無需複雜熱循環的情況下進行擴增,從而使設備更易於在分散測試和資源有限的環境中使用
- 與數位健康平台和基於雲端的數據管理系統的整合正在增強結果報告、遠端監控和流行病學監測能力
- 這一趨勢正在改變分子診斷,實現分散式檢測,縮短週轉時間,並提高已開發市場和新興市場的可近性
- BioFire Diagnostics 和 Qiagen 等公司正在積極推動具有多重檢測能力和用戶友好介面的便攜式核酸擴增解決方案,以滿足醫療保健和公共衛生領域對快速現場診斷日益增長的需求
核酸擴增市場動態
司機
傳染病和個人化醫療導致需求增加
- 全球傳染病的盛行率不斷上升,包括新出現的病毒爆發和抗生素抗藥性細菌感染,這是核酸擴增技術的重要驅動力,該技術可以提供靈敏且特異的病原體檢測
- 例如,2024 年,羅氏診斷擴大了其分子檢測產品組合,增加了針對傳染源的新檢測方法,從而能夠更快地識別和管理疾病
- 個人化醫療和癌症及罕見疾病基因檢測的日益普及,進一步推動了對支持標靶治療和診斷的精確核酸擴增方法的需求
- 醫療保健基礎設施投資的增加(尤其是在發展中國家)以及政府為提高診斷能力而採取的措施促進了市場的成長
- 臨床、研究和法醫應用對快速、準確、經濟高效的分子診斷的需求繼續推動市場的擴張
克制/挑戰
技術複雜性和監管障礙
- 核酸擴增技術的複雜性和診斷檢測審批的嚴格監管要求對市場成長和產品商業化提出了挑戰
- 先進設備、試劑和技術人員的高成本可能會限制其採用,特別是在資源匱乏的環境中
- 例如,2023 年,一家領先的診斷公司因嚴格的驗證要求而面臨 FDA 對新型 PCR 檢測方法的批准延遲,影響了其市場准入時間
- 此外,與污染風險、檢測靈敏度和可重複性相關的問題需要持續的品質控制和驗證,這增加了實驗室的運作負擔
- 透過技術簡化、成本降低、簡化監管途徑和強有力的品質保證來克服這些挑戰對於更廣泛的市場滲透和持續成長至關重要
核酸擴增市場範圍
市場根據應用和技術進行細分。
- 按類型
根據應用,核酸擴增市場細分為傳染病、癌症、個人化醫療和基因檢測。傳染病領域佔據市場主導地位,2024年收入份額最高,達52.5%,這得益於臨床診斷和疫情管理中對快速準確的病原體檢測的迫切需求。全球傳染病的高發病率和持續的病毒疫情是維持該領域強勁需求的關鍵因素。
預計癌症領域將在2025年至2032年期間實現最快的複合年增長率,這得益於分子診斷在早期癌症檢測、突變分析和治療監測中的日益普及。個人化醫療也因人們日益關注基於基因圖譜的標靶治療而快速發展。在遺傳性疾病篩檢和研究應用的推動下,基因檢測正在穩步發展。
- 依技術
根據技術,核酸擴增市場可細分為標靶擴增、探針擴增和訊號擴增。標靶擴增領域佔據市場主導地位,2024年的收入份額約為57%,這主要得益於PCR及相關方法的廣泛應用,這些方法可擴增特定的DNA或RNA序列,實現靈敏且特異的檢測。
探針擴增技術預計將在2025年至2032年間實現最快的複合年增長率,因為它透過放大探針訊號而非目標序列來增強檢測特異性,這在某些需要高精度的診斷應用中非常有用。訊號擴增技術預計將得到更廣泛的應用,因為它能夠在不擴增目標核酸本身的情況下提高檢測靈敏度,這在複雜樣本基質和多重檢測中非常有用。
核酸擴增市場區域分析
- 北美在核酸擴增市場佔據主導地位,2024 年的收入份額最大,為 39.4%,這得益於先進的醫療基礎設施、高額的研發投入以及診斷檢測和平台領域關鍵參與者的創新
- 該地區的醫療保健提供者和研究機構優先考慮對傳染病、癌症和遺傳疾病進行快速、準確的診斷,並得到廣泛的研發活動和主要行業參與者的支持
- 該地區完善的監管框架和對個人化醫療日益增長的重視進一步加速了核酸擴增檢測的普及,使北美成為臨床和研究領域創新分子診斷解決方案的領先市場
美國核酸擴增市場洞察
2024年,美國核酸擴增市場佔據北美地區最大收入份額,達39%,這得益於先進分子診斷技術的快速普及以及對快速、精準傳染病檢測需求的不斷增長。即時診斷平台的廣泛應用,加上醫療研發和數位整合投資的不斷增加,進一步推動了市場的成長。此外,核酸擴增檢測與人工智慧數據分析和電子健康記錄的整合,顯著提高了診斷效率和療效。
歐洲核酸擴增市場洞察
預計歐洲核酸擴增市場在整個預測期內將穩步增長,這得益於嚴格的監管標準、傳染病發病率的上升以及對早期癌症檢測和基因檢測需求的不斷增長。德國和英國等國家在政府加大對分子診斷的投入以及醫療數位化程度的提升的推動下,市場成長強勁。該地區注重高品質診斷和個人化醫療,這推動了核酸擴增技術在臨床和研究機構中的應用。
英國核酸擴增市場洞察
英國核酸擴增市場預計在預測期內將以顯著的複合年增長率擴張,這得益於基因組研究投資的增加和精準醫療應用的持續推進。針對傳染病控制和癌症篩檢的公共衛生措施正在推動對核酸擴增檢測的需求。英國蓬勃發展的生物技術產業和強大的醫療基礎設施進一步刺激了成長,醫院和診所越來越多地採用即時分子診斷技術。
德國核酸擴增市場洞察
預計德國核酸擴增市場將迎來顯著成長,這得益於其對醫療創新和數位轉型的高度重視。德國重視早期疾病診斷和預防性醫療保健,鼓勵採用先進的核酸擴增技術。此外,嚴格的資料隱私和安全法規也催生了對安全可靠的診斷解決方案的需求。在德國臨床實驗室中,自動化實驗室系統和智慧資料管理平台的整合正變得越來越普遍。
亞太核酸擴增市場洞察
受醫療支出成長、傳染病負擔加重以及中國、印度和日本等國家分子診斷應用不斷擴大的推動,亞太地區核酸擴增市場預計在2025年至2032年期間以約22%的複合年增長率快速增長。政府推動數位化醫療的舉措、個人化醫療意識的提升以及生物技術基礎設施的不斷擴展,正在加速市場成長。此外,該地區還受益於試劑和儀器的本地化生產,從而提高了核酸擴增解決方案的可負擔性和可及性。
日本核酸擴增市場洞察
日本核酸擴增市場的成長得益於其先進的醫療體系、人口老化以及對技術創新的高度重視。慢性病盛行率的上升以及分子診斷在癌症和遺傳性疾病管理中的日益普及,刺激了市場需求。核酸擴增與物聯網和人工智慧驅動的健康監測系統的整合也提高了日本診斷的效率和準確性。
印度核酸擴增市場洞察
2024年,印度核酸擴增市場佔據亞太地區最大收入份額,這得益於快速城鎮化、醫療基礎設施擴張以及政府對傳染病控制日益重視。中產階級人口的不斷增長和分子診斷意識的不斷提升,推動了公共和私人醫療機構對分子診斷技術的採用。對經濟實惠的診斷解決方案的追求以及國內生產能力的不斷提升,進一步加速了印度市場的成長。
核酸擴增市佔率
核酸擴增產業主要由知名公司主導,包括:
- 西門子(德國)
- Hologic公司(美國)
- 深圳邁瑞生物醫療電子股份有限公司 (中國)
- 雅培(美國)
- BD(美國)
- F. Hoffmann-La Roche Ltd(瑞士)
- 賽默飛世爾科技公司(美國)
- Koninklijke Philips NV(荷蘭)
- NeuroLogica Corp.(美國)
- 島津醫療(印度)私人有限公司(日本)
- 通用電氣(美國)
- Quest Diagnostics Incorporated(美國)
- 希森美康印度列兵。株式會社(日本)
- 日立有限公司(日本)
- 佳能公司(日本)
- 富士膠片控股公司(英國)
- Illumina公司(美國)
- 丹納赫公司(美國)
- Bio-Rad Laboratories, Inc.(美國)
- 諾華公司(瑞士)
- Seegene Inc.(韓國)
全球核酸擴增市場的最新發展是什麼?
- 2025 年 6 月,生物梅里埃公司發布了一種用於檢測細胞和基因治療產品中支原體的自動化核酸擴增檢測方法,該檢測方法基於等溫技術,提供高通量的內部解決方案,只需極少的技術工作,即可在約 1 小時內提供結果
- 2025 年 5 月,QIAGEN NV 簽署最終協議,以 8,000 萬美元(包括 7,000 萬美元現金以及最高 1,000 萬美元的或有付款)收購 Genoox, Ltd.,旨在增強其臨床基因組學和分子診斷產品
- 2025年5月,一項新發表的研究展示了一種逆轉錄重組酶聚合酶擴增 (RT-RPA) 檢測方法,結合側流檢測 (LFD) 平台,可快速、靈敏且特異地檢測登革熱病毒血清型2 (DENV2)。此檢測方法的檢測極限約為每次反應50個拷貝,與其他常見病原體無交叉反應,並且在各種環境條件下都能可靠地進行檢測。
- 2025 年 4 月,研究人員發布了基於 PCR 的開源即時診斷平台,該平台在檢測呼吸道病毒和 HPV 病毒方面具有 100% 的靈敏度和 98% 以上的特異性,ROC AUC 值超過 0.98,使其成為一種高度準確且低成本的分散診斷替代方案
- 2025 年 4 月,Meridian Bioscience, Inc. 推出了用於分子診斷的新型酶穩定服務,涵蓋 qPCR、等溫擴增和 NGS,旨在提高試劑穩定性、實現室溫格式、簡化工作流程並降低成本
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。