Global Clinical Trials Market
市场规模(十亿美元)
CAGR :
% 
 USD
297.62 Billion
USD
444.77 Billion
2024
2032
USD
297.62 Billion
USD
444.77 Billion
2024
2032
| 2025 –2032 | |
| USD 297.62 Billion | |
| USD 444.77 Billion | |
|
|
|
|
全球臨床試驗市場細分,按階段(I 期、II 期、III 期和 IV 期)、適應症(自體免疫/發炎、疼痛管理、腫瘤學、中樞神經系統疾病、糖尿病、肥胖症、心血管疾病等)、設計(介入、治療研究、觀察性研究和擴展訪問)、最終用戶(醫院、實驗室和診所)——行業趨勢和預測到 2032 年。
臨床試驗市場規模
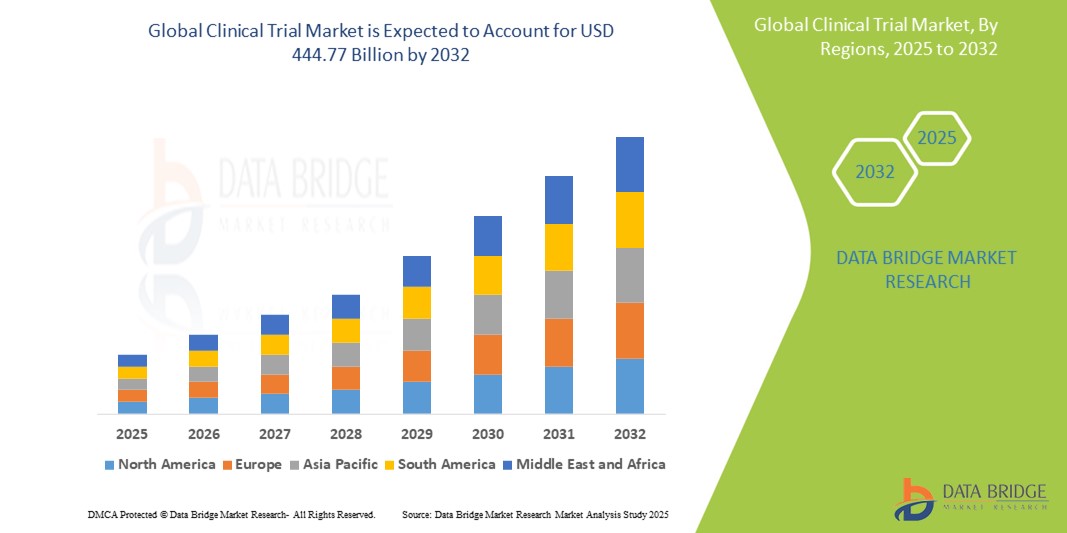
- 2024 年全球臨床試驗市場價值為2.9762 億美元,預計到 2032 年將達到 4,447.7 億美元
- 在 2025 年至 2032 年的預測期內,市場可能會以5.15% 的複合年增長率增長,主要原因是對創新療法的需求不斷增長、臨床研究技術的進步以及臨床試驗外包的興起
- 這種增長受到多種因素的推動,例如臨床試驗的日益複雜化、臨床試驗數量的增加、分散試驗的不斷普及以及對成本效益高且更快速的試驗流程的需求不斷增加
臨床試驗市場分析
- 臨床試驗是一項涉及人類參與者的研究,旨在評估新醫療治療或介入措施的安全性、有效性和潛在副作用
- 全球臨床試驗市場正在迅速擴張,這得益於人們越來越重視開發新療法,並透過新療法和新藥物改善患者護理,尤其是在腫瘤學和罕見疾病等領域
- 例如,用於癌症治療的 CAR-T 細胞療法的開發顯著加速了腫瘤學的臨床試驗
- 使用人工智慧和機器學習進行數據分析等技術進步顯著提高了臨床試驗的效率和準確性
- 例如,在臨床試驗患者招募中使用人工智慧,IBM Watson Health 等公司正在努力簡化流程並確保更快的參與者匹配
- 全球範圍內進行的臨床試驗數量增加,新冠疫苗臨床試驗是該產業快速適應緊急公共衛生需求的典型案例。輝瑞和Moderna新冠疫苗快速的臨床試驗流程凸顯了該產業在壓力下加速研發的能力。
- 將臨床試驗外包給合約研究組織 (CRO) 正變得越來越普遍,這有助於製藥公司降低成本並獲得專業知識。一個實際案例是諾華 (Novartis) 與科文斯 (Covance) 在全球臨床試驗方面的合作,科文斯 (Covance) 為管理大規模國際試驗提供了專業服務。
- 分散式試驗的整合正在加速,允許患者透過數位平台在家中參與,尤其是在疫情之後
- 例如,阿斯特捷利康新冠疫苗臨床試驗採用分散試驗模式,以提高參與度並維持安全規程
報告範圍和臨床試驗市場細分
|
屬性 |
臨床試驗關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
北美洲
歐洲
亞太
中東和非洲
南美洲
|
|
主要市場參與者 |
|
|
市場機會 |
|
|
加值資料資訊集 |
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。 |
臨床試驗市場趨勢
“分散式臨床試驗的採用日益廣泛”
- 分散式臨床試驗使患者能夠在家中或當地醫療機構參與,減少了頻繁訪問中心研究地點的需要,從而提高了參與者的便利性
- 例如,輝瑞在新冠疫情期間進行的遠端試驗允許患者無需前往試驗現場即可參與疫苗研究
- 這些試驗利用遠距醫療、穿戴式裝置和數位平台等技術來監測患者、收集數據和保持溝通,從而可以即時追蹤患者的健康狀況
- 例如,禮來公司在糖尿病藥物臨床試驗中使用穿戴式裝置遠端監控患者。
- 新冠疫情加速了分散臨床試驗的採用,醫療保健產業也隨之調整以確保連續性
- 例如,Moderna 對其 COVID-19 疫苗研發進行了遠端試驗,利用遠距醫療和居家監控來追蹤疫苗接種者,同時確保安全並降低暴露風險
- 分散式試驗消除了前往臨床地點的長途障礙,增加了患者就診機會,尤其是在農村或醫療資源匱乏的地區
- 例如,阿斯特捷利康的新冠疫苗試驗採用了虛擬諮詢和藥物送貨上門的方式,讓偏遠地區的患者也能繼續參與試驗
- 製藥公司和合約研究組織現在採用混合模式,將傳統的現場試驗與分散方法結合
- 例如,諾華公司在其正在進行的全球腫瘤治療試驗中加入了分散元素,允許偏遠地區的患者參與,同時仍遵守試驗方案
臨床試驗市場動態
司機
“對新型創新療法的需求不斷增長”
- 對新型和創新療法的需求不斷增長,尤其是在腫瘤學、罕見疾病和慢性病領域,正在推動臨床試驗市場的發展
- 例如,CAR-T 細胞療法等個人化癌症治療的興起,促使人們進行大量試驗來測試其療效和安全性
- 製藥公司和研究機構正專注於標靶治療和精準醫療,這需要嚴格的臨床試驗
- 例如,脊髓性肌肉萎縮症等基因療法的開發,已經經過了廣泛的臨床試驗,以確保其有效性
- 癌症免疫檢查點抑制劑等突破性療法(包括 Keytruda 和 Opdivo 等藥物)加速了臨床試驗的需求,以改善和擴大其應用,目前許多試驗正在研究其在各種癌症類型中的應用
- 包括輝瑞和 Moderna 在內的 COVID-19 疫苗的快速研發,凸顯了快速臨床試驗的迫切需要,展示了臨床研究如何快速適應公共衛生需求並加快提供挽救生命的治療
- 慢性病和人口老化的日益普及促使臨床試驗的增加
- 例如,全球糖尿病發生率的不斷上升促使人們進行大量試驗,探索新的治療方法和技術,以改善患者的治療效果。
機會
“新興市場對臨床試驗的需求不斷增長”
- 印度和中國等新興市場的臨床試驗需求不斷增長,這些市場的患者群體龐大且多樣化,因此招募速度更快,測試條件也更加多樣化
- 例如,在印度,由於擁有大量且多樣化的患者群體,疫苗和癌症治療試驗一直在擴大
- 全球慢性病和人口老化日益加劇,導致對更多療法的需求增加,並擴大了腫瘤學和糖尿病等領域的臨床試驗機會。糖尿病病例的增加促使美國和歐洲等國家開展了更多臨床試驗,以測試新的胰島素製劑和治療方案。
- 數位平台和虛擬試驗等技術進步,簡化了招募、資料收集和監控,同時降低了試驗成本
- 例如,Science 37 等平台已經實現了遠距臨床試驗,讓患者在家中參與試驗,並減少了旅行需求
- 擴大巴西和韓國等國家的監管彈性,加速試驗審批速度,並鼓勵國際製藥公司在這些地區進行研究。巴西的監管機構-國家衛生監督局(ANVISA)簡化了臨床試驗審批流程,使其成為拉丁美洲臨床研究的重要目的地。
- 更重視個人化醫療,從而促進了針對特定患者亞群的精準試驗的發展,例如罕見疾病基因療法的興起
- 例如,針對脊髓性肌肉萎縮症的基因療法 Zolgensma 的臨床試驗,該療法針對受影響兒童的特定基因標記進行定制
克制/挑戰
“進行臨床試驗的複雜性”
- 各國的指導方針不同,導致監理挑戰,審批流程複雜化
- 例如,由於美國 FDA 和歐洲 EMA 的規定不同,COVID-19 疫苗試驗最近被推遲,這表明國際合規性有多複雜
- 病患招募和保留往往因認知度和可及性有限而受到阻礙,尤其是在服務不足的地區
- 例如,杜氏肌肉營養不良症等罕見疾病的臨床試驗面臨招募足夠合格參與者的挑戰,從而延遲了研發
- 隨著試驗產生大量訊息,數據管理變得越來越困難。在多中心試驗中,確保資料收集的準確性和一致性(例如在腫瘤學試驗中),需要先進的技術來防止錯誤,正如阿斯特捷利康疫苗試驗中資料不一致導致的短暫挫折所證明的那樣。
- 成本和時間限制仍然是臨床試驗中的一個主要問題,尤其是在那些涉及長期或大量患者追蹤的臨床試驗中。進行大規模試驗(例如針對阿茲海默症藥物的試驗)的成本可能超過數百萬美元,而且延誤也很常見,例如阿杜卡努單抗(Aducanumab)的試驗延誤。
- 圍繞病人安全和知情同意的倫理考量至關重要,尤其是在涉及弱勢群體時。基因療法的臨床試驗,例如脊髓性肌肉萎縮症的臨床試驗,需要仔細的倫理考量,以確保患者充分了解其風險,Zolgensma 的審批流程就是一個例證。
臨床試驗市場範圍
市場根據階段、適應症、設計和最終用戶進行細分
|
分割 |
細分 |
|
按階段 |
|
|
按適應症 |
|
|
按設計 |
|
|
按最終用戶 |
|
臨床試驗市場區域分析
“北美是臨床試驗市場的主導地區”
- 北美在臨床試驗市場佔據主導地位,這主要歸功於美國強大的醫療保健體系和先進的技術,為臨床試驗提供了堅實的基礎
- 該地區是許多全球製藥和生物技術公司的所在地,推動創新和臨床研究
- 北美受益於清晰且支持性的監管流程,這些流程有利於臨床試驗的批准和執行
- 持續的臨床研究資金以及龐大、多樣化的患者群體加速了市場成長並增強了北美的主導地位
“亞太地區預計將實現最高成長率”
- 亞太地區是臨床試驗成長最快的市場,這得益於快速擴張的醫療保健市場對創新治療和藥物的需求
- 具有吸引力臨床試驗目的地,尤其是中國、印度和日本,因為這些國家人口基數大、種類多樣,且成本比西方市場低
- 醫療保健基礎設施的改善和臨床研究站點數量的增加促進了該地區的快速增長
- 更有利的監管環境,支持該地區臨床試驗的有效發展
- 醫療保健領域的持續投資和越來越多的經驗豐富的專業人員,使亞太地區成為臨床試驗領域成長最快的地區
臨床試驗市場佔有率
市場競爭格局按競爭對手提供詳細資料。詳細資訊包括公司概況、公司財務狀況、收入、市場潛力、研發投入、新市場計劃、全球影響力、生產基地和設施、生產能力、公司優勢和劣勢、產品發布、產品寬度和廣度以及應用主導地位。以上提供的數據點僅與公司在市場中的重點相關。
市場中主要的市場領導者有:
- Clinipace(美國)
- 美國實驗室控股公司(LabCorp)(美國)
- 禮來公司(美國)
- ICON Plc.(愛爾蘭)
- 諾和諾德公司(丹麥)
- Parexel International Corporation(美國)
- 輝瑞公司(美國)
- PPD公司(美國)
- IQVIA(美國)
- 賽諾菲(法國)
- F. Hoffmann-La Roche Ltd(瑞士)
- Alcami Corporation, Inc.(美國)
- Accell Clinical Research LLC(美國)
- Congenix LLP(英國)
- Labcorp藥物開發(美國)
- Ecron Acunova(印度)
- Medpace(美國)
- LUMITOS AG(德國)
- ICON plc(愛爾蘭)
- SIRO Clapham Private Limited(印度)
全球臨床試驗市場的最新發展
- 2025年2月,Novotech與原州塞弗倫斯基督教醫院合作,共同推動韓國臨床研究的卓越發展。此次合作旨在利用該醫院先進的醫療基礎設施和專業知識,提升臨床試驗能力。此次合作將專注於改善韓國的臨床研究服務,並擴大臨床試驗機會。預計此次合作將提高臨床試驗的速度和效率,並透過提供更強大、更可靠的研究環境,使韓國和國際製藥公司均能從中受益。此次合作將對市場產生重大影響,因為它將使韓國成為更具吸引力的全球臨床試驗目的地,並有可能加速亞太地區臨床研究市場的成長。
- 2025年1月,ICON plc宣布開發其基於人工智慧的工具組合,以提高臨床試驗效率。該公司全新的人工智慧解決方案旨在簡化臨床試驗的各個環節,包括病患招募、數據分析和營運管理。這項進步將縮短試驗時間、降低成本並改善整個試驗流程的決策。其優點包括優化資源利用率、更好地定位患者以及提高試驗結果的準確性。這些人工智慧驅動的工具將對市場產生巨大影響,因為這些工具將改變臨床試驗格局,使全球製藥公司能夠更有效率、更具成本效益、更具可擴展性。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。