Global Adalimumab Biosimilar Market
市场规模(十亿美元)
CAGR :
% 
| 2023 –2030 | |
| USD 598.30 Million | |
| USD 3,431.48 Million | |
|
|
|
>全球阿达木单抗生物仿制药市场,按产品(Exemptia、Adalirel、Cipleumab、其他)、适应症(类风湿性关节炎、幼年特发性关节炎、银屑病关节炎、其他适应症)、分销渠道(医院药房、零售药房、网上药房、其他)– 行业趋势和预测到 2030 年。
阿达木单抗生物仿制药市场分析和规模
根据 GLOBOCAN 2020,2020 年美国将新增 2,281,658 例癌症病例,死亡人数将近 612,390 人。此外,主要市场参与者预测,在预测期内,市场将通过各种战略活动(如产品发布、合并和收购)实现增长。例如,2021 年 7 月,FDA 批准了 Viatris Inc.(前身为 Mylan Pharmaceuticals Inc.)的 SEMGLEE(甘精胰岛素-yfgn),这是 LANTUS(甘精胰岛素)的生物仿制药。
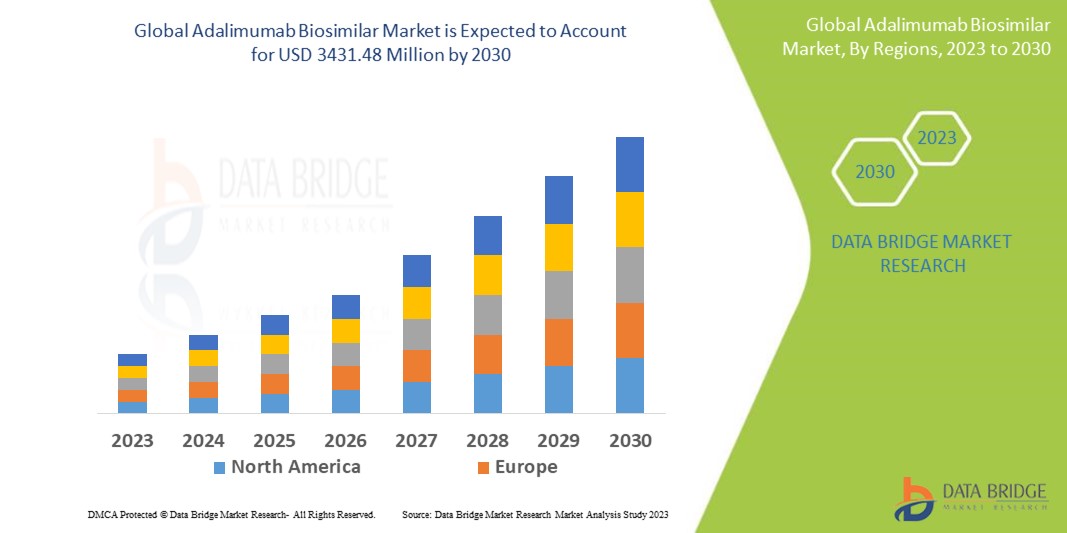
Data Bridge Market Research 分析,阿达木单抗生物仿制药市场在 2022 年的规模为 5.983 亿美元,预计到 2030 年将达到 34.3148 亿美元,在 2023 年至 2030 年的预测期内复合年增长率为 24.4%。除了对市场价值、增长率、细分、地理覆盖范围和主要参与者等市场情景的见解外,Data Bridge Market Research 策划的市场报告还包括深度专家分析、患者流行病学、管道分析、定价分析和监管框架。
阿达木单抗生物仿制药市场范围和细分
|
报告指标 |
细节 |
|
预测期 |
2023 至 2030 年 |
|
基准年 |
2021 |
|
历史岁月 |
2020(可定制为 2014 - 2019) |
|
定量单位 |
收入(百万美元)、销量(单位)、定价(美元) |
|
涵盖的领域 |
产品(Exemptia、Adalirel、Cipleumab、其他)、分销渠道(医院药房、零售药房、其他) |
|
覆盖国家 |
北美洲的美国、加拿大和墨西哥、德国、法国、英国、荷兰、瑞士、比利时、俄罗斯、意大利、西班牙、土耳其、欧洲其他地区、中国、日本、印度、韩国、新加坡、马来西亚、澳大利亚、泰国、印度尼西亚、菲律宾、亚太地区 (APAC) 的其他地区、沙特阿拉伯、阿联酋、南非、埃及、以色列、中东和非洲 (MEA) 的其他地区、巴西、阿根廷和南美洲其他地区 |
|
涵盖的市场参与者 |
Alfred E. Tiefenbacher (GmbH & Co. KG)(德国)、安进公司(美国)、勃林格殷格翰国际有限公司(德国)、Glenmark(印度)、Zydus Group(印度)、Torrent Pharmaceuticals Ltd.(印度)、Reliance Life Sciences(印度)、Emcure Pharmaceuticals Ltd(印度)、Cipla Inc.(印度)、Hetero(印度)、AET BioTech(德国)、Coherus Biosciences(美国)、富士胶片协和麒麟生物制剂有限公司(日本)、Momenta Pharmaceuticals(美国)、Oncobiologics(美国)、辉瑞公司(美国)、三星生物肽(韩国)、Sandoz International GmbH(瑞士) |
|
Market Opportunities |
|
Market Definition
Adalimumab, a monoclonal antibody, is used to treat autoimmune diseases such as rheumatoid arthritis and ulcerative colitis, among others. This medication helps to prevent future joint damage and maintain joint function by reducing joint swelling. As the global prevalence of arthritis rises, the market for adalimumab biosimilars is expected to grow during the forecast period.
Adalimumab Biosimilar Market Dynamics
Drivers
- Rise in biologic drugs
Several blockbuster biologic drugs from major pharmaceutical companies, including Truvada, Chantix, Forteo, Ciprodex, Afinitor, and many others, will lose US exclusivity in 2020. Several existing biological drugs, such as Erbitux, Avastin, and Orencia, will have their patents expire in the coming decade, providing an opportunity for many innovator companies as well as generic manufacturers to offer services specifically tailored toward biosimilars. Furthermore, the cost-effective nature of biosimilars, rising acceptance and adoption by various stakeholders, the need for diversification in technology and business models, and the growing prevalence of chronic diseases are expected to drive the global biosimilar market.
Opportunities
- Rising cancer cases
Cancer's growing burden and rising death toll necessitate the need for affordable treatment, boosting the growth of the biosimilar market. According to GLOBOCAN 2020, there will be 2,281,658 new cancer cases and nearly 612,390 deaths in the United States in 2020. Furthermore, key market participants predicted market growth during the forecast period through a variety of strategic activities such as product launches, mergers, and acquisitions. For instance, in July 2021, the FDA approved Viatris Inc.'s (formerly Mylan Pharmaceuticals Inc.) SEMGLEE (insulin-glargine-yfgn), a biosimilar to LANTUS (insulin glargine).
Restraints/Challenges
- High cost
The high cost associated with biosimilars will obstruct the market's growth rate. However, the global adalimumab biosimilar market is hampered by a scarcity of skilled specialists. Furthermore, factors such as high temperature sensitivity, lack of efficacy and safety, and high manufacturing costs associated with generic drugs are expected to limit market growth between 2022 and 2030.
This adalimumab biosimilar market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the adalimumab biosimilar market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Adalimumab Biosimilar Market Scope
The adalimumab biosimilar market is segmented on the basis of product and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Exemptia
- Adalirel
- Cipleumab
- Others
Distribution Channel
- Hospitals Pharmacies
- Retail Pharmacies
- Others
Adalimumab Biosimilar Market Regional Analysis/Insights
The adalimumab biosimilar market is analyzed and market size insights and trends are provided by country, product and distribution channel as referenced above.
The countries covered in the adalimumab biosimilar market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the adalimumab biosimilar market due to the proximity of multiple extensive experimentation laboratories.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 because of the province's extensive commercial advancements and growing biotechnology corporations.
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化会影响市场的当前和未来趋势。下游和上游价值链分析、技术趋势和波特五力分析、案例研究等数据点是用于预测单个国家市场情景的一些指标。此外,在提供国家数据的预测分析时,还考虑了全球品牌的存在和可用性以及它们因来自本地和国内品牌的激烈或稀缺竞争而面临的挑战、国内关税和贸易路线的影响。
竞争格局和阿达木单抗生物仿制药市场份额分析
阿达木单抗生物仿制药市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、收入、市场潜力、研发投资、新市场计划、全球影响力、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度、应用主导地位。以上提供的数据点仅与公司对阿达木单抗生物仿制药市场的关注有关。
阿达木单抗生物仿制药市场的一些主要参与者包括:
- Alfred E. Tiefenbacher (GmbH & Co. KG)(德国)
- 安进公司 (美国)
- 勃林格殷格翰国际有限公司 (德国)
- 格兰马克(印度)
- Zydus 集团 (印度)
- Torrent Pharmaceuticals Ltd. (印度)
- Reliance Life Sciences(印度)
- Emcure Pharmaceuticals Ltd (印度)
- Cipla Inc.(印度)
- 异性恋(印度)
- AET BioTech(德国)
- Coherus Biosciences(美国)
- 富士胶片协和麒麟生物制品有限公司(日本)
- Momenta Pharmaceuticals (美国)
- 肿瘤生物学(美国)
- 辉瑞公司(美国)
- 三星 Bioepsis (韩国)
- Sandoz International GmbH(瑞士)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
可定制
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.