Asia Pacific Medical Devices Market
市场规模(十亿美元)
CAGR :
% 
 USD
5.52 Billion
USD
9.01 Billion
2024
2032
USD
5.52 Billion
USD
9.01 Billion
2024
2032
| 2025 –2032 | |
| USD 5.52 Billion | |
| USD 9.01 Billion | |
|
|
|
|
亞太醫療器材市場細分,按產品(重建關節置換、脊椎植入物、創傷和顱顎面、牙科植入物和骨科生物製品)、設備類型(內固定裝置和外固定裝置)、生物材料(金屬生物材料、聚合物生物材料、陶瓷生物材料、天然生物材料等)、手術(開放手術、微創手術 (MIS))和最終用戶(診所 203 年行業中心
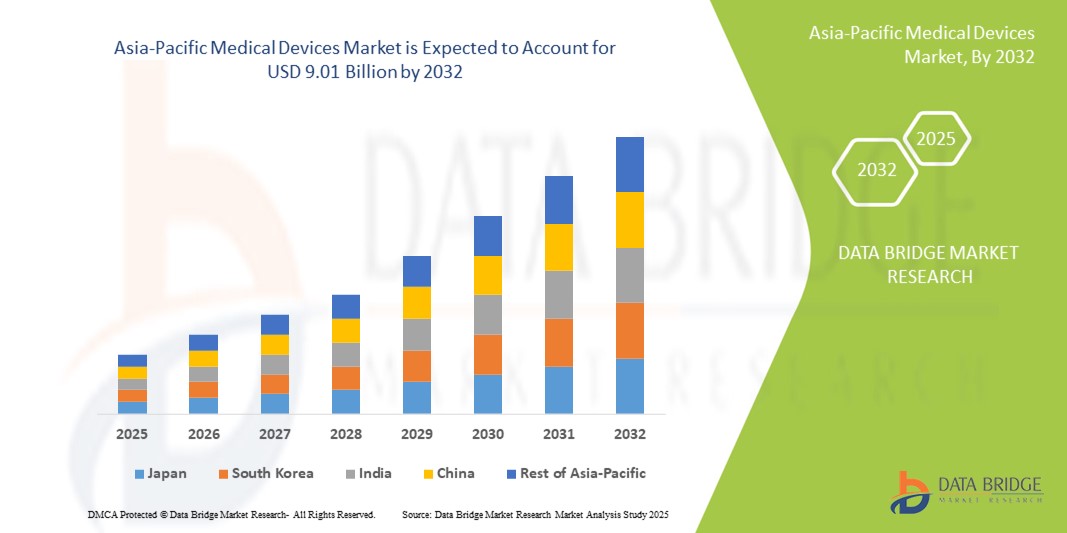
亞太醫療器材市場規模
- 2024 年亞太醫療器材市場規模為55.2 億美元 ,預計 到 2032 年將達到 90.1 億美元,預測期內 複合年增長率為 6.30%。
- 市場成長主要得益於亞太地區新興經濟體快速的城市化、不斷擴大的醫療基礎設施以及醫療診斷和治療設備的技術進步,從而提高了醫療服務體系的可及性和現代化程度
- 此外,對價格實惠、便攜且微創的醫療器材的需求不斷增長,加上政府投資的增加和有利的監管改革,正在使亞太地區成為全球醫療器材市場的關鍵成長中心。這些因素正在加速創新醫療技術的普及,從而顯著推動中國、印度、日本和韓國等國家的醫療器材產業成長。
亞太醫療器材市場分析
- 由於慢性病盛行率上升、醫療保健覆蓋率不斷擴大以及對早期準確診斷的日益重視,包括診斷、治療和監測設備在內的醫療器材在亞太地區正變得越來越重要。科技的快速進步以及公共和私營部門醫療保健支出的增加,進一步加速了醫院、診所和家庭護理機構對創新醫療器材的採用。
- 亞太地區醫療器材需求激增,主要源自於人口老化、呼吸系統和心血管疾病發生率上升,以及對高效居家照護解決方案的普遍需求。慢性阻塞性肺病 (COPD)、氣喘和睡眠呼吸中止症等疾病的盛行率不斷上升,刺激了呼吸器、持續性呼吸道正壓通氣 (CPAP)/雙水平氣道正壓通氣 (BIPAP) 和製氧機等設備的需求。
- 中國在亞太醫療器材市場佔據主導地位,2024年市佔率高達39.6%,這得益於其龐大的病患群體、快速發展的醫療數位化以及國內生產能力強勁、價格合理且先進的醫療技術。政府支持農村醫療現代化和慢性病篩檢計畫的措施也是關鍵因素。
- 預計印度將成為2025年至2032年間亞太醫療器材市場成長最快的地區。醫療保健投資的增加、私立醫院的擴張、「印度製造」等政府舉措以及人們對關節和創傷相關治療的認識不斷提高等因素,正在顯著推動市場成長。
- 由於骨關節炎和類風濕性關節炎發病率不斷上升,以及需要進行膝關節和髖關節置換手術的老年人口不斷增加,重建關節置換手術在亞太醫療器械市場佔據主導地位,2024 年其收入份額最高,為 32.8%。
報告範圍和亞太醫療器材市場細分
|
屬性 |
亞太醫療器材關鍵市場洞察 |
|
涵蓋的領域 |
|
|
覆蓋國家 |
亞太
|
|
主要市場參與者 |
|
|
市場機會 |
|
|
加值資料資訊集 |
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深入的專家分析、定價分析、品牌份額分析、消費者調查、人口統計分析、供應鏈分析、價值鏈分析、原材料/消耗品概述、供應商選擇標準、PESTLE 分析、波特分析和監管框架。 |
亞太醫療器材市場趨勢
“對智慧互聯醫療保健解決方案的需求不斷增長”
- 亞太醫療器材市場一個顯著且加速發展的趨勢是人工智慧 (AI) 和物聯網 (IoT) 等先進技術的深度融合,旨在提升各種設備的功能性、準確性和使用者體驗。這些創新正在簡化醫療保健服務,並在臨床和家庭環境中實現即時數據驅動的決策。
- 例如,人工智慧呼吸器和便攜式氧氣濃縮器正被廣泛採用,可根據患者生命徵象自動調整呼吸支援。同樣,智慧型CPAP/BiPAP設備正在提供即時回饋和依從性跟踪,使患者和醫療服務提供者都能優化治療效果。
- 醫療設備與行動應用程式和雲端平台的整合,實現了遠端患者監控、早期診斷和預防性護理,這在農村或醫療服務匱乏的地區尤其有益。這種數位轉型正在亞太地區建構一個更互聯的醫療保健生態系統。
- 實現醫療設備和電子健康記錄 (EHR) 之間集中控制和互通性的技術也提高了工作流程效率,並減輕了醫院和診所的管理負擔
- 日益普及的用戶友善智慧醫療設備正在重塑患者和醫療服務提供者的期望。因此,該地區的公司正在開發更智慧、更便利的醫療設備,並配備自動警報、遠端調整和健康追蹤儀錶板等功能。
- 受醫療保健意識的提高、慢性病負擔的增加以及政府對數位健康計劃的支持推動,亞太地區對綜合數據驅動醫療解決方案的需求正在迅速增長
亞太醫療器材市場動態
司機
“醫療保健需求和技術應用不斷增長,需求也隨之增長”
- 慢性病負擔加重、人口老化以及改善醫療基礎設施的需求是推動亞太地區加速採用先進醫療設備的關鍵因素。快速的城市化和醫療保健意識的提升進一步推動政府和私營部門投資於現代化的診斷和治療設備。
- 例如,2024年3月,日本厚生勞動省批准撥款,用於下一代呼吸器和便攜式診斷設備,以加強居家護理和疫情防治。預計公共機構和私人企業的此類舉措將在預測期內推動亞太醫療器材市場的成長。
- 患者和醫療服務提供者越來越意識到早期診斷和預防保健的好處,推動了便攜式氧氣濃縮器、數位肺量計和 AI 整合 CPAP/BiPAP 機器等技術的採用,這些技術可為患者提供更佳的治療效果
- 此外,遠距醫療服務的日益普及以及去中心化照護的趨勢,使得醫療設備在居家照護環境中變得更加重要。能夠輕鬆與行動醫療平台和電子健康記錄 (EHR) 整合的設備正成為遠端患者監控的必備工具。
- 醫療設備向便攜、用戶友好且高效的轉變,正在推動大型醫院和小型診所的廣泛採用。隨著醫療保險覆蓋範圍的擴大以及政府對數位醫療的支持,醫療設備在印度、中國和東南亞等國家越來越普及。
克制/挑戰
“對監管複雜性和高初始成本的擔憂”
- 亞太醫療器材市場面臨各國監管架構差異所帶來的挑戰,這可能會阻礙產品審批和市場准入。製造商必須應對中國、印度和日本等市場不同的合規要求,這增加了營運的複雜性和成本。
- 例如,2021 年中國新實施的《醫療器材法規》(MDR)增加了對臨床證據的要求,這可能會延遲產品上市並增加開發成本
- 此外,呼吸器、麻醉機和診斷影像系統等先進醫療設備的高前期成本,可能會對發展中地區的小型醫療機構和機構造成阻礙。預算限制和融資管道的匱乏,限制了這些設備在農村和服務欠缺地區的廣泛應用。
- 儘管價格正在逐漸下降,本地製造也在增加,但高端醫療設備的高成本仍然令人擔憂,特別是對於長期照護和小型醫療機構而言
- 克服這些挑戰需要協調監管流程、增加對本地製造業的投資、政府補貼和宣傳活動,以提高人們對新醫療技術的可負擔性和信任度
亞太醫療器材市場範圍
市場根據產品、設備類型、生物材料、程序和最終用戶進行細分。
• 按產品
亞太醫療器材市場按產品細分為重建關節置換、脊椎植入物、創傷及顱顎面、牙科植入物、骨科生物製品。重建關節置換領域佔市場主導地位,2024年收入份額最高,達32.8%,這得益於骨關節炎和類風濕性關節炎發病率的上升,以及需要膝關節和髖關節置換的老年人口的不斷增長。
受牙科旅遊業的蓬勃發展、口腔健康意識的增強以及植牙材料和技術的進步的推動,預計 2025 年至 2032 年間,植牙領域將實現最快的複合年增長率,達到 24.1%。
• 依設備類型
根據設備類型,亞太醫療器材市場分為內固定器材和外固定器材。內固定器械在2024年佔據了最大的市場份額,達到58.5%,這是由於骨折治療和脊椎固定中對內固定的偏好較高,這有助於更好的癒合並縮短住院時間。
預計外固定裝置領域將以穩定的複合年增長率成長,這得益於創傷護理和骨科急救幹預應用的增加。
• 依生物材料
根據生物材料,亞太醫療器材市場細分為金屬生物材料、聚合物生物材料、陶瓷生物材料、天然生物材料及其他。金屬生物材料在2024年佔據最大份額,為41.3%,這歸因於其優異的強度和耐用性,廣泛應用於關節置換和脊椎固定等承重植入物。
由於聚合物生物材料在組織工程、藥物傳輸和生物可吸收設備中的應用日益廣泛,預計其複合年增長率將達到 22.6%。
• 依程序
根據手術方式,亞太醫療器材市場可分為開放性手術和微創手術(MIS)。 2024年,開放手術佔最大份額,達56.4%,這主要得益於其在複雜骨科重建和創傷護理中的持續應用。
預計從 2025 年到 2032 年,微創手術 (MIS) 將以 27.8% 的最快複合年增長率增長,這得益於其縮短恢復時間、減輕術後疼痛和提高手術精度等優勢。
• 按最終用戶
根據最終用戶,亞太醫療器材市場可細分為醫院、門診護理中心、專科診所、骨科中心及其他。醫院憑藉其全面的服務、先進的基礎設施以及更高的外科手術患者人次,在2024年以48.9%的最高收入份額領先市場。
預計骨科中心將以最快的複合年增長率成長,這得益於該地區發展中國家越來越多專門的骨科和復健設施的建立。
亞太醫療器材市場區域分析
- 亞太地區在全球醫療器材市場佔據主導地位,2024 年其收入份額最大,為 34.80%,這得益於醫療支出的增加、快速的城市化以及需要骨科和診斷幹預的老年人口的不斷擴大
- 地區政府正在大力投資改善醫療基礎設施,而醫療旅遊業的興起,尤其是在印度、泰國和馬來西亞等國家,進一步刺激了對先進醫療技術的需求
- 人們對微創手術的認識不斷提高、醫療設施的可及性不斷提高以及優惠的報銷政策也是該地區市場成長的主要推動因素
中國亞太醫療器材市場洞察
2024年,中國醫療器材市場在亞太地區佔據最大份額,達39.6%,這得益於中國龐大的人口基數、日益增長的老年人口以及骨科手術需求的增加。國內製造商的策略性舉措以及政府的醫療改革措施,加速了尖端醫療器材的普及和價格的可負擔性。
日本亞太醫療器材市場洞察
日本醫療器材市場持續成長,得益於其雄厚的技術基礎、人口老化以及對高品質精準醫療解決方案的強烈需求。日本致力於創新,並率先採用先進的外科手術技術,促進了微創和重建器械的使用率的提升。
印度亞太醫療器材市場洞察
預計印度醫療器材市場將在 2025 年至 2032 年間經歷最快的複合年增長率。醫療保健投資增加、私人醫院擴張、「印度製造」等政府舉措以及人們對關節和創傷相關治療的認識不斷提高等因素正在極大地推動市場成長。
亞太醫療器材市場份額
亞太醫療器材 產業主要由知名公司主導,包括:
- Zimmer Biomet(美國)
- Smith + Nephew(英國)
- 美敦力(愛爾蘭)
- 史賽克(美國)
- B. Braun SE(德國)
- NuVasive, Inc.(美國)
- ENOVIS公司(美國)
- Institut Straumann AG(瑞士)
- OSSTEM IMPLANT CO., LTD.(韓國)
- Narang Medical Limited(美國)
- Globus Medical(美國)
- Arthrex, Inc.(美國)
- CONMED公司(美國)
- Integra LifeSciences Corporation(美國)
- RTI 外科(美國)
- 戈爾公司(美國)
- Corin集團(英國)
- 強生服務公司(美國)
亞太醫療器材市場最新動態
- 2024年5月,施樂輝公司在馬來西亞開設了一家新的研發和製造中心,以支持亞太地區對骨科和傷口護理產品日益增長的需求。該中心增強了公司的區域能力,彰顯了其致力於擴大東南亞地區先進醫療器材的可及性並提高本地生產效率的承諾。
- 2024年4月,史賽克在日本和澳洲的多家醫院推出了Mako智慧機器人系統,實現了精準、微創的關節置換手術。此舉鞏固了史賽克在亞太地區的影響力,並展現了機器人輔助手術技術在亞太地區醫療保健系統中日益普及的趨勢。
- 2024年3月,美敦力與印度阿波羅醫院合作,擴大其Micra AV心律調節器的使用範圍。 Micra AV心律調節器是一款專為房室傳導阻滯治療而設計的微型設備。此次合作滿足了印度日益增長的創新心臟護理需求,並體現了美敦力致力於改善新興市場患者治療效果的策略。
- 2024年2月,捷邁邦美宣佈在韓國部分醫院推出其 Persona IQ 智慧膝關節系統。該設備將骨科植入物與智慧感測器技術相結合,為外科醫生和患者提供術後監測和復健的即時數據洞察。
- 2024年1月,費雪派克醫療保健 (Fisher & Paykel Healthcare) 在中國推出 Airvo 3 高流量呼吸機,拓展了其呼吸照護產品線,以滿足該地區日益增長的非侵入性氧氣治療解決方案需求。此舉彰顯了公司對創新的重視,並致力於為亞太市場提供量身定制的醫療保健解決方案。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA-PACIFIC MEDICAL DEVICE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL SWINE AND POULTRY RESPIRATORY DISEASES TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 ASIA-PACIFIC MEDICAL DEVICE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR ASIA-PACIFIC MEDICAL DEVICE MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY PRODUCT TYPE
16.1 OVERVIEW
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS OF PRODUCTS)
16.2 RESPIRATORY DEVICES
16.2.1 THERAPEUTIC
16.2.1.1. VENTILATOR
16.2.1.1.1. MARKET VALUE (USD)
16.2.1.1.2. MARKET VOLUME (UNIT)
16.2.1.1.3. ASP (USD)
16.2.1.2. MASK
16.2.1.3. PAP DEVICE
16.2.1.4. INHALER
16.2.1.5. NEB
16.2.1.6. ULIZER
16.2.2 MONITORING
16.2.2.1. PULSE OXIMETER
16.2.2.1.1. MARKET VALUE (USD)
16.2.2.1.2. MARKET VOLUME (UNIT)
16.2.2.1.3. ASP (USD)
16.2.3 CAPNOGRAPH
16.2.3.1. MARKET VALUE (USD)
16.2.3.2. MARKET VOLUME (UNIT)
16.2.3.3. ASP (USD)
16.2.4 DIAGNOSTIC
16.2.4.1. MARKET VALUE (USD)
16.2.4.2. MARKET VOLUME (UNIT)
16.2.4.3. ASP (USD)
16.2.5 CONSUMABLES
16.2.5.1. MARKET VALUE (USD)
16.2.5.2. MARKET VOLUME (UNIT)
16.2.5.3. ASP (USD)
16.3 DIAGNOSTIC DEVICES
16.3.1 ELECTRODIAGNOSTIC DEVICE
16.3.1.1. ULTRASOUND SYSTEMS
16.3.1.2. MAGNETIC RESONANCE IMAGING (MRI)
16.3.1.3. ELECTROCARDIOGRAPHS
16.3.1.4. SCINTIGRAPHIC APPARATUS
16.3.1.5. OTHER ELECTRODIAGNOSTIC DEVICE
16.3.2 RADIATION DEVICE
16.3.2.1. SMARTWATCHES WITH HEALTH MONITORING
16.3.2.2. WEARABLE BLOOD PRESSURE MONITORS
16.3.2.3. REMOTE MONITORING SYSTEMS
16.3.2.4. TELEMEDICINE DEVICES
16.3.2.5. CT SCANNER16.3.2.6.
16.3.3 IMAGING PARTS & ACCESSORIES
16.3.3.1. CONTRAST MEDIA
16.3.3.2. X-RAY TUBES
16.3.3.3. MEDICAL X-RAY FILM16.3.3.4.
16.4 CARDIOVASCULAR DEVICES
16.4.1 ELECTROCARDIOGRAM (ECG)
16.4.1.1. REMOTE CARDIAC MONITORING
16.4.1.2. OTHER DIAGNOSTIC AND MONITORING DEVICE
16.4.2 THERAPEUTIC AND SURGICAL DEVICE
16.4.2.1. CARDIAC ASSIST DEVICE
16.4.2.2. CARDIAC RHYTHM MANAGEMENT DEVICE
16.4.2.3. CATHETER
16.4.2.4. GRAFTS
16.4.2.5. HEART VALVES
16.4.2.6. STENTS
16.4.2.7. OTHER THERAPEUTIC AND SURGICAL DEVICE
16.5 DENTAL
16.5.1 DENTAL INSTRUMENT & SUPPLIES
16.5.1.1. DENTAL INSTRUMENTS
16.5.1.2. DENTAL CEMENTS
16.5.1.3. TEETH & OTHER FITTINGS
16.5.2 DENTAL CAPITAL EQUIPMENT
16.5.2.1. DENTAL DRILLS
16.5.2.2. DENTAL X-RAY
16.5.2.3. DENTAL CHAIRS
16.6 ORTHOPEDIC DEVICES
16.6.1 FIXATION DEVICE
16.6.2 ARTIFICIAL JOINTS
16.6.3 OTHER ARTIFICIAL BODY PARTS
16.7 ENDOSCOPY DEVICES
16.7.1 VISUALIZATION EQUIPMENT
16.7.1.1. ENDOSCOPIC CAMERA
16.7.1.2. SD VISUALIZATION SYSTEM
16.7.1.3. HD VISUALIZATION SYSTEM
16.7.2 ENDOSCOPES
16.7.2.1. RIGID ENDOSCOPE
16.7.2.2. FLEXIBLE ENDOSCOPE
16.7.2.3. CAPSULE ENDOSCOPE
16.7.2.4. ROBOT-ASSISTED ENDOSCOPE
16.7.3 ENDOSCOPIC OPERATIVE DEVICE
16.7.3.1. IRRIGATION/SUCTION SYSTEM
16.7.3.2. ACCESS DEVICE
16.7.3.3. WOUND PROTECTOR
16.7.3.4. INSUFFLATION DEVICE
16.7.3.5. OPERATIVE MANUAL INSTRUMENT
16.7.3.6. OTHER ENDOSCOPIC OPERATIVE DEVICE
16.8 OPHTHALMOLOGY DEVICES
16.9 RADIOTHERAPY DEVICES
16.1 AESTHETIC DEVICES
16.10.1 LABORATORY EQUIPMENT
16.10.1.1. GENERAL EQUIPMENT
16.10.1.2. INCUBATORS
16.10.1.3. CENTRIFUGES
16.10.1.4. LABORATORY HOOD
16.10.1.5. AUTOCLAVE
16.10.1.6. SCOPES
16.10.1.7. SONICATORS
16.10.1.8. OTHERS
16.11 ANALYTICAL EQUIPMENT
16.11.1 SPECTROMETER
16.11.1.1. MASS SPECTROMETER
16.11.1.2. FLUORESCENCE SPECTROMETER
16.11.1.3. INFRARED SPECTROMETER
16.11.1.4. OTHERS
16.11.2 ANALYZER
16.11.2.1. ELEMENTAL ANALYZERS
16.11.2.2. PARTICLE SIZE ANALYZERS
16.11.2.3. OTHERS
16.11.3 TITRATORS
16.11.4 RHEOMETERS
16.11.5 FLOW INJECTION SYSTEM
16.11.6 SAMPLE PREPARATION SYSTEM
16.11.7 CHROMATOGRAPHY EQUIPMENT
16.11.7.1. GAS CHROMATOGRAPHY EQUIPMENT
16.11.7.2. LIQUID CHROMATOGRAPHY EQUIPMENT
16.11.8 OTHERS
16.12 SUPPORT EQUIPMENT
16.12.1 CELL HARVESTERS
16.12.2 RADIOMETRIC DETECTORS
16.12.3 MICROPLATE READERS
16.12.4 OTHERS
16.13 SPECIALTY EQUIPMENT
16.13.1 CYTOGENETICS INSTRUMENTS
16.13.2 CELL IMAGING DEVICE
16.13.3 LABORATORY EVAPORATORS
16.13.4 POLARIMETERS
16.13.5 MEMBRANE FILTRATION SYSTEMS
16.13.6 LASER SYSTEMS
16.13.7 OTHERS
16.14 SPECTROMETERS
16.15 OTHERS
17 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY DEVICE CLASS
17.1 CLASS I
17.2 CLASS II
17.3 CLAS III
17.4 CLASS IV
18 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY TYPE
18.1 INVASIVE DEVICE
18.2 NON-INVASIVE DEVICE
18.3 IMPLANTABLE DEVICE
18.4 WEARABLE DEVICE
18.5 OTHERS
19 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY APPLICATION
19.1 DIAGNOSTIC
19.2 THERAPEUTIC
20 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY MANUFACTURING METHOD
20.1 IN-HOUSE MANUFACTURING
20.2 OUTSOURCING
21 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS & CLINICS
21.2.1 IN-PATIENT
21.2.2 OUT-PATIENT
21.3 SPECIALTY CLINICS
21.4 AMBULATORY CARE CENTERS
21.5 BIOPHARMACEUTICAL COMPANIES
21.6 LABORATORIES
21.7 ACADEMICS & RESEARCH INSTITUTES
21.8 OTHERS
22 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 DISTRIBUTORS
22.4 RETAIL SALES
22.5 OTHERS
23 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY GEOGRAPHY
ASIA-PACIFIC MEDICAL DEVICE MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 ASIA-PACIFIC
23.1.1 JAPAN
23.1.2 CHINA
23.1.3 SOUTH KOREA
23.1.4 INDIA
23.1.5 AUSTRALIA
23.1.6 SINGAPORE
23.1.7 THAILAND
23.1.8 MALAYSIA
23.1.9 INDONESIA
23.1.10 PHILIPPINES
23.1.11 REST OF ASIA-PACIFIC
23.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 ASIA-PACIFIC MEDICAL DEVICE MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 ASIA-PACIFIC MEDICAL DEVICE MARKET, SWOT AND DBMR ANALYSIS
26 ASIA-PACIFIC MEDICAL DEVICE MARKET, COMPANY PROFILE
26.1 MEDICAL DEVICE
26.1.1 MEDTRONIC PLC.
26.1.1.1. COMPANY OVERVIEW
26.1.1.2. REVENUE ANALYSIS
26.1.1.3. GEOGRAPHIC PRESENCE
26.1.1.4. PRODUCT PORTFOLIO
26.1.1.5. RECENT DEVELOPMENTS
26.1.2 BOSTON SCIENTIFIC CORPORATION
26.1.2.1. COMPANY OVERVIEW
26.1.2.2. REVENUE ANALYSIS
26.1.2.3. GEOGRAPHIC PRESENCE
26.1.2.4. PRODUCT PORTFOLIO
26.1.2.5. RECENT DEVELOPMENTS
26.1.3 JOHNSON & JOHNSON
26.1.3.1. COMPANY OVERVIEW
26.1.3.2. REVENUE ANALYSIS
26.1.3.3. GEOGRAPHIC PRESENCE
26.1.3.4. PRODUCT PORTFOLIO
26.1.3.5. RECENT DEVELOPMENTS
26.1.4 BECTON, DICKINSON AND COMPANY
26.1.4.1. COMPANY OVERVIEW
26.1.4.2. REVENUE ANALYSIS
26.1.4.3. GEOGRAPHIC PRESENCE
26.1.4.4. PRODUCT PORTFOLIO
26.1.4.5. RECENT DEVELOPMENTS
26.1.5 CARDINAL HEALTH
26.1.5.1. COMPANY OVERVIEW
26.1.5.2. REVENUE ANALYSIS
26.1.5.3. GEOGRAPHIC PRESENCE
26.1.5.4. PRODUCT PORTFOLIO
26.1.5.5. RECENT DEVELOPMENTS
26.1.6 STRYKER CORPORATION
26.1.6.1. COMPANY OVERVIEW
26.1.6.2. REVENUE ANALYSIS
26.1.6.3. GEOGRAPHIC PRESENCE
26.1.6.4. PRODUCT PORTFOLIO
26.1.6.5. RECENT DEVELOPMENTS
26.1.7 ABBOTT LABORATORIES
26.1.7.1. COMPANY OVERVIEW
26.1.7.2. REVENUE ANALYSIS
26.1.7.3. GEOGRAPHIC PRESENCE
26.1.7.4. PRODUCT PORTFOLIO
26.1.7.5. RECENT DEVELOPMENTS
26.1.8 BAXTER INTERNATIONAL
26.1.8.1. COMPANY OVERVIEW
26.1.8.2. REVENUE ANALYSIS
26.1.8.3. GEOGRAPHIC PRESENCE
26.1.8.4. PRODUCT PORTFOLIO
26.1.8.5. RECENT DEVELOPMENTS
26.1.9 DANAHER CORPORATION
26.1.9.1. COMPANY OVERVIEW
26.1.9.2. REVENUE ANALYSIS
26.1.9.3. GEOGRAPHIC PRESENCE
26.1.9.4. PRODUCT PORTFOLIO
26.1.9.5. RECENT DEVELOPMENTS
26.1.10 3M
26.1.10.1. COMPANY OVERVIEW
26.1.10.2. REVENUE ANALYSIS
26.1.10.3. GEOGRAPHIC PRESENCE
26.1.10.4. PRODUCT PORTFOLIO
26.1.10.5. RECENT DEVELOPMENTS
26.1.11 NOVARTIS AG
26.1.11.1. COMPANY OVERVIEW
26.1.11.2. REVENUE ANALYSIS
26.1.11.3. GEOGRAPHIC PRESENCE
26.1.11.4. PRODUCT PORTFOLIO
26.1.11.5. RECENT DEVELOPMENTS
26.1.12 GENERAL ELECTRIC COMPANY
26.1.12.1. COMPANY OVERVIEW
26.1.12.2. REVENUE ANALYSIS
26.1.12.3. GEOGRAPHIC PRESENCE
26.1.12.4. PRODUCT PORTFOLIO
26.1.12.5. RECENT DEVELOPMENTS
26.1.13 BD
26.1.13.1. COMPANY OVERVIEW
26.1.13.2. REVENUE ANALYSIS
26.1.13.3. GEOGRAPHIC PRESENCE
26.1.13.4. PRODUCT PORTFOLIO
26.1.13.5. RECENT DEVELOPMENTS
26.1.14 INTUITIVE SURGICAL
26.1.14.1. COMPANY OVERVIEW
26.1.14.2. REVENUE ANALYSIS
26.1.14.3. GEOGRAPHIC PRESENCE
26.1.14.4. PRODUCT PORTFOLIO
26.1.14.5. RECENT DEVELOPMENTS
26.1.15 ALLERGAN
26.1.15.1. COMPANY OVERVIEW
26.1.15.2. REVENUE ANALYSIS
26.1.15.3. GEOGRAPHIC PRESENCE
26.1.15.4. PRODUCT PORTFOLIO
26.1.15.5. RECENT DEVELOPMENTS
26.1.16 HOYA CORPORATION
26.1.16.1. COMPANY OVERVIEW
26.1.16.2. REVENUE ANALYSIS
26.1.16.3. GEOGRAPHIC PRESENCE
26.1.16.4. PRODUCT PORTFOLIO
26.1.16.5. RECENT DEVELOPMENTS
26.1.17 SIEMENS HEALTHCARE GMBH
26.1.17.1. COMPANY OVERVIEW
26.1.17.2. REVENUE ANALYSIS
26.1.17.3. GEOGRAPHIC PRESENCE
26.1.17.4. PRODUCT PORTFOLIO
26.1.17.5. RECENT DEVELOPMENTS
26.1.18 RESMED
26.1.18.1. COMPANY OVERVIEW
26.1.18.2. REVENUE ANALYSIS
26.1.18.3. GEOGRAPHIC PRESENCE
26.1.18.4. PRODUCT PORTFOLIO
26.1.18.5. RECENT DEVELOPMENTS
26.1.19 TERUMO MEDICAL CORPORATION
26.1.19.1. COMPANY OVERVIEW
26.1.19.2. REVENUE ANALYSIS
26.1.19.3. GEOGRAPHIC PRESENCE
26.1.19.4. PRODUCT PORTFOLIO
26.1.19.5. RECENT DEVELOPMENTS
26.1.20 OLYMPUS CORPORATION
26.1.20.1. COMPANY OVERVIEW
26.1.20.2. REVENUE ANALYSIS
26.1.20.3. GEOGRAPHIC PRESENCE
26.1.20.4. PRODUCT PORTFOLIO
26.1.20.5. RECENT DEVELOPMENTS
26.1.21 ZIMMER BIOMET
26.1.21.1. COMPANY OVERVIEW
26.1.21.2. REVENUE ANALYSIS
26.1.21.3. GEOGRAPHIC PRESENCE
26.1.21.4. PRODUCT PORTFOLIO
26.1.21.5. RECENT DEVELOPMENTS
26.1.22 FESENIUS MEDICAL CARE
26.1.22.1. COMPANY OVERVIEW
26.1.22.2. REVENUE ANALYSIS
26.1.22.3. GEOGRAPHIC PRESENCE
26.1.22.4. PRODUCT PORTFOLIO
26.1.22.5. RECENT DEVELOPMENTS
26.1.23 EDWARDS LIFESCIENCES CORPORATION
26.1.23.1. COMPANY OVERVIEW
26.1.23.2. REVENUE ANALYSIS
26.1.23.3. GEOGRAPHIC PRESENCE
26.1.23.4. PRODUCT PORTFOLIO
26.1.23.5. RECENT DEVELOPMENTS
26.1.24 KONINKLIJKE PHILIPS N.V.
26.1.24.1. COMPANY OVERVIEW
26.1.24.2. REVENUE ANALYSIS
26.1.24.3. GEOGRAPHIC PRESENCE
26.1.24.4. PRODUCT PORTFOLIO
26.1.24.5. RECENT DEVELOPMENTS
26.1.25 DRÄGERWERK AG & CO. KGAA
26.1.25.1. COMPANY OVERVIEW
26.1.25.2. REVENUE ANALYSIS
26.1.25.3. GEOGRAPHIC PRESENCE
26.1.25.4. PRODUCT PORTFOLIO
26.1.25.5. RECENT DEVELOPMENTS
26.1.26 COLOPLAST GROUP
26.1.26.1. COMPANY OVERVIEW
26.1.26.2. REVENUE ANALYSIS
26.1.26.3. GEOGRAPHIC PRESENCE
26.1.26.4. PRODUCT PORTFOLIO
26.1.26.5. RECENT DEVELOPMENTS
26.1.27 WATERS CORPORATION
26.1.27.1. COMPANY OVERVIEW
26.1.27.2. REVENUE ANALYSIS
26.1.27.3. GEOGRAPHIC PRESENCE
26.1.27.4. PRODUCT PORTFOLIO
26.1.27.5. RECENT DEVELOPMENTS
26.1.28 HOLOGIC, INC.
26.1.28.1. COMPANY OVERVIEW
26.1.28.2. REVENUE ANALYSIS
26.1.28.3. GEOGRAPHIC PRESENCE
26.1.28.4. PRODUCT PORTFOLIO
26.1.28.5. RECENT DEVELOPMENTS
26.1.29 STERIS
26.1.29.1. COMPANY OVERVIEW
26.1.29.2. REVENUE ANALYSIS
26.1.29.3. GEOGRAPHIC PRESENCE
26.1.29.4. PRODUCT PORTFOLIO
26.1.29.5. RECENT DEVELOPMENTS
26.1.30 INSTITUT STRAUMANN AG
26.1.30.1. COMPANY OVERVIEW
26.1.30.2. REVENUE ANALYSIS
26.1.30.3. GEOGRAPHIC PRESENCE
26.1.30.4. PRODUCT PORTFOLIO
26.1.30.5. RECENT DEVELOPMENTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。