慢性病是持续时间较长的非传染性疾病,需要持续的医疗护理和管理。
根据世界卫生组织的数据,慢性病占全球死亡人数的近 70%,其中心血管疾病是主要原因。人口老龄化、久坐的生活方式、营养不良和环境因素等多种因素导致慢性病患病率不断上升。放射性药物可以帮助医疗保健提供者更高效、更有效地管理慢性病。
不同类型的慢性疾病包括癌症、心血管疾病和糖尿病等,这些疾病在欧洲的发病率正在上升。由于人们久坐不动的生活方式,高血压和糖尿病等生活方式相关疾病的发病率有所上升。因此,随着医疗保健数据与便携式设备的日益融合,慢性病的发病率不断上升,预计需要适当的健康管理,从而导致预测期内欧洲市场对放射性药物的需求预计会增加。
访问完整报告@https://www.databridgemarketresearch.com/reports/europe-radiopharmaceuticals-market
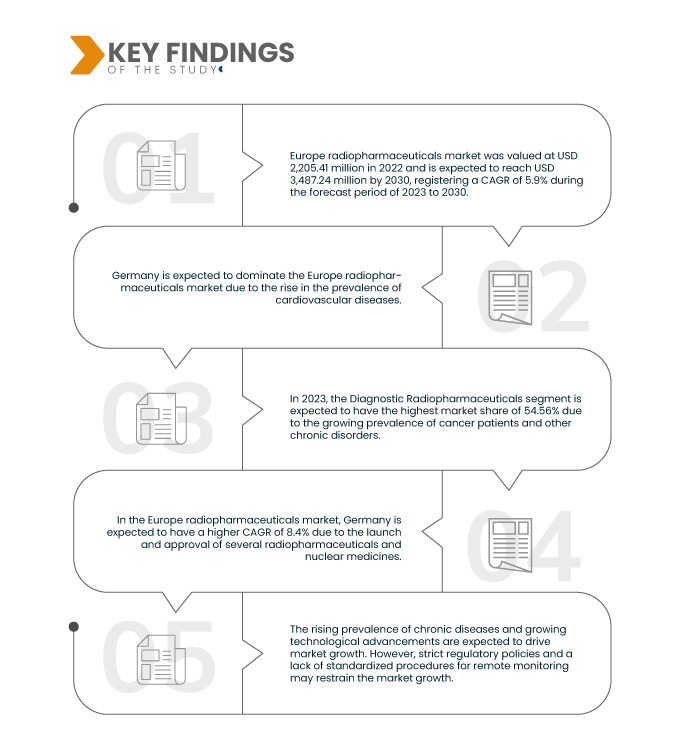
Data Bridge Market Research 分析称, 欧洲放射性药物市场 预计 2023 年至 2030 年的复合年增长率为 5.9%,到 2030 年预计将达到 34.8724 亿美元。
研究主要发现
诊断和临床应用对放射性药物的需求不断增长
放射性药物已成为帮助诊断器官和治疗病理疾病的必不可少的药物。它们用于诊断成像和 放射治疗. 诊断放射性药物不具有药理作用,并且其使用也不被认为与相关的临床副作用有关。
临床使用的放射性药物数量正在迅速增加,从而使医学界能够更好地获取有关不同类型肿瘤特征的详细信息。放射性核素也因对越来越多的恶性疾病提供姑息和治愈治疗而变得越来越重要。
放射性药物用于诊断癌症等复杂疾病,并且已被证明在临床应用中是有效的,这增加了其需求并有望推动市场增长。
报告范围和市场细分
|
报告指标
|
细节
|
|
预测期
|
2023 至 2030 年
|
|
基准年
|
2022
|
|
历史性的一年
|
2021(可定制为 2015 – 2020)
|
|
定量单位
|
收入(百万美元)
|
|
涵盖的领域
|
类型(诊断放射性药物和治疗放射性药物)、应用(诊断和治疗)、来源(核反应堆和回旋加速器)、最终用户(医院、诊断中心、癌症研究机构、门诊手术中心等)
|
|
覆盖国家
|
德国、英国、法国、意大利、西班牙、比利时、波兰、俄罗斯、挪威、土耳其、奥地利、爱尔兰、匈牙利、荷兰、瑞士和欧洲其他地区
|
|
涵盖的市场参与者
|
Cardinal Health(美国)、Advanced Accelerator Applications(诺华公司)(法国)、Lantheus(美国)、CURIUM(法国)(美国)、Jubilant Radiopharma(Jubilant Pharma 公司)(印度)、中国同位素辐照有限公司(中国)、西门子医疗有限公司(德国)、Bracco(美国)、Eckert & Ziegler(德国)、SHINE Technologies, LLC(美国)和 IBA Worldwide(比利时)等
|
|
报告中涵盖的数据点
|
除了对市场价值、增长率、细分、地理覆盖范围和主要参与者等市场情景的洞察之外,Data Bridge Market Research 策划的市场报告还包括深度专家分析、竞争分析、品牌分析、技术趋势、渠道分析、定价分析和监管框架。
|
细分分析
欧洲放射性药物市场根据类型、来源、应用和最终用户分为四个显著的部分。
- 根据类型,市场分为诊断放射性药物和治疗放射性药物。
2023 年,诊断放射性药物领域预计将主导欧洲放射性药物市场。
到 2023 年,由于慢性病发病率不断上升以及医生和患者越来越多地采用核医学进行治疗和诊断,诊断放射性药物领域预计将占据市场主导地位,市场份额为 54.56%。
- 根据应用,市场分为诊断和治疗。到 2023 年,诊断领域预计将占据市场主导地位,市场份额为 51.91%。
- 根据来源,市场分为核反应堆和回旋加速器。 2023 年,核反应堆部分预计将占据市场主导地位,市场份额为 57.91%。
- 根据最终用户,市场细分为医院、诊断中心、癌症研究机构、 门诊手术中心, 和别的。
到 2023 年,医院部门预计将主导欧洲放射性药物市场。
到 2023 年,由于慢性病患者越来越多地采用放射性药物来诊断和治疗癌症和心血管疾病患者,预计医院部门将以 35.67% 的市场份额占据市场主导地位。
主要参与者
Cardinal Health(美国)、Advanced Accelerator Applications、诺华公司(法国)、Lantheus(美国)、CURIUM(法国)、通用电气公司(美国)等。
市场发展
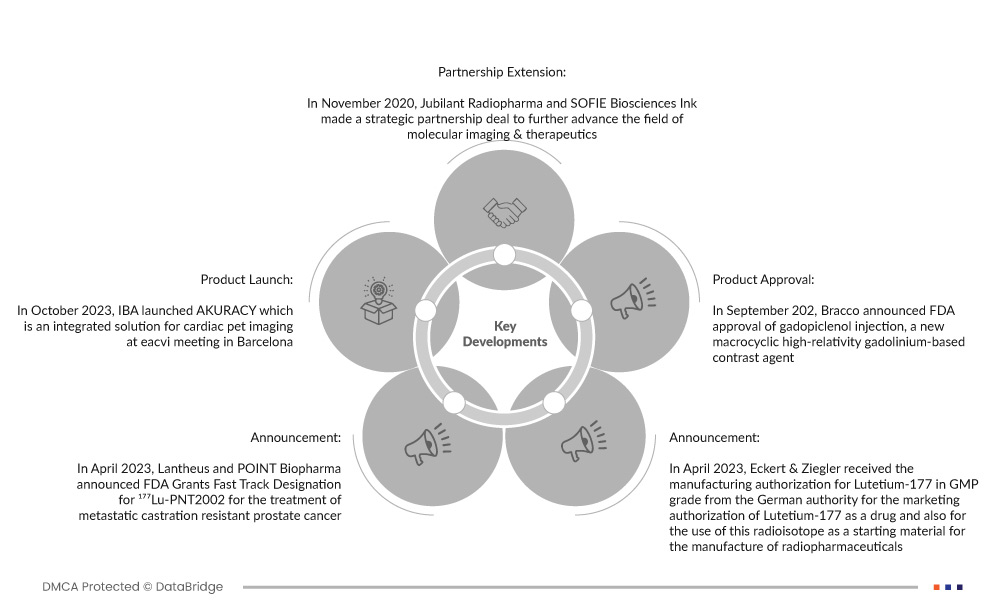
- 2023年10月,IBA在巴塞罗那的eacvi会议上推出了心脏PET成像集成解决方案AKURACY。该公司相信并表示,这是PET心肌灌注成像的独特集成解决方案。
- 2023 年 4 月,Lantheus 和 POINT Biopharma 宣布 FDA 授予 ¹⁷⁷Lu-PNT2002 快速通道资格,用于治疗转移性去势抵抗性前列腺癌。该公司表示,放射性配体疗法正迅速成为癌症治疗的另一个支柱。
- 2023 年 4 月,Eckert & Ziegler 从德国主管部门获得了 GMP 级镥-177 的生产许可,该部门授权镥-177 作为药物进行营销,并授权使用该放射性同位素作为制造放射性药物的起始材料。该公司认为这可能有助于治疗神经内分泌肿瘤和前列腺癌。
- 2022 年 12 月,诺华获批 Pluvicto 作为首个用于治疗前列腺癌的靶向放射性配体疗法。该公司认为,这将有助于推进欧洲治疗癌症的广泛放射性配体疗法组合。
- 2022 年 9 月,Bracco 宣布 FDA 批准了一种新型大环高相对性钆基造影剂 gadopiclenol 注射剂。该公司认为,这将有助于在成人和 2 岁及以上的儿科患者中使用 MRI 来检测和观察中枢神经系统的病变。
- 2020 年 11 月,Jubilant Radiopharma 与 SOFIE Biosciences Ink 达成战略合作协议,以进一步推进分子成像和治疗领域。该公司认为,这将有助于提高生产能力,也将有助于推进其治疗诊断管道。
区域分析
从地理上看,欧洲放射性药物市场报告涵盖的国家包括德国、英国、法国、意大利、西班牙、比利时、波兰、俄罗斯、挪威、土耳其、奥地利、爱尔兰、匈牙利、荷兰、瑞士和欧洲其他地区。
根据 Data Bridge 市场研究分析:
德国是欧洲最强、速度最快的国家 市场 在 2023 年至 2030 年的预测期内。
由于整个地区放射性药物的采用率不断提高,德国预计将占据主导地位并成为增长最快的国家。放射性药物供应量的不断增加以及对所有慢性和非慢性疾病的患者进行监测的做法的不断增加预计将推动该地区的市场增长。
有关放射性药物市场的更多详细信息,请点击此处 –https://www.databridgemarketresearch.com/reports/europe-radiopharmaceuticals-market