胃腸道疾病的日益普及推動了對糞便診斷的需求。胃腸道疾病可引起許多症狀,包括腹瀉、便秘、腹痛和噁心。各種情況,包括感染和發炎性腸道疾病,都可能導致這些症狀。糞便檢查可以檢測出細菌、病毒和寄生蟲等傳染源以及發炎的生物標記。除了對透過糞便診斷胃腸道疾病的需求不斷增長之外,人們對使用糞便測試監測腸道微生物群的興趣也日益濃厚。糞便測試可以提供有關微生物群組成和功能的信息,這有助於識別菌群失調(微生物群失衡)並指導幹預措施以改善腸道健康。
訪問完整報告@ https://www.databridgemarketresearch.com/reports/asia-pacific-stool-diagnostic-market
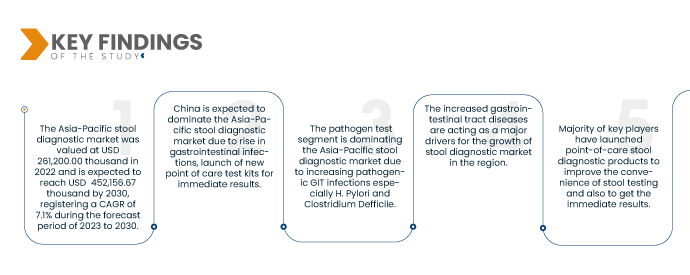
Data Bridge Market Research 分析稱,亞太地區糞便診斷市場預計在 2023 年至 2030 年期間的複合年增長率為 7.1%,到 2030 年將達到 452,156.67 萬美元。人們越來越意識到糞便診斷在診斷胃腸道疾病的重要性,預計將推動市場的成長。
研究的主要發現
食道癌、大腸癌和胃癌發生率不斷上升
食道癌、大腸癌和胃癌的發生率不斷上升,推動了對糞便診斷的需求。這些類型的癌症會影響消化系統,並可能導致排便習慣發生很大變化。
胃癌也是一個嚴重的健康問題,尤其是在亞洲,胃癌更為常見。糞便檢測可以檢測出與胃癌相關的生物標記物,例如幽門螺旋桿菌(H. pylori)感染,這是胃癌的主要原因。
糞便診斷是早期發現和監測此類癌症的重要工具。糞便檢查可以檢測出癌症相關生物標記的存在,例如血液、DNA和蛋白質。這可以實現更早的發現和乾預,改善患者的治療效果並減輕此類癌症的負擔。糞便檢查可以檢測出顯示存在癌症的生物標記物,例如糞便中的血液(可能是結腸直腸癌的徵兆)或腫瘤 DNA(可能是食道癌或胃癌的徵兆)。
食道癌在亞洲特別常見,是導致癌症死亡的主要原因。糞便測試可以檢測與食道癌相關的生物標記物,例如 DNA 突變和腫瘤特異性蛋白質。在食道癌盛行率較高的亞洲,使用糞便檢測進行食道癌篩檢和監測變得越來越重要。
糞便潛血檢測 (FOBT) 和糞便免疫化學檢測 (FIT) 通常用於大腸直腸癌篩檢,可檢測與疾病相關的血液和其他生物標記。糞便 DNA 檢測可以檢測出與大腸直腸癌相關的特定 DNA 突變,這種檢測也越來越普及。
報告範圍和市場細分
報告指標
|
細節
|
預測期
|
2023年至2030年
|
基準年
|
2022
|
歷史歲月
|
2021(可自訂為2015-2020)
|
定量單位
|
收入(千美元),定價(美元)
|
涵蓋的領域
|
依檢測類型(糞便潛血檢測、糞便生物標記檢測、病原體檢測、糞便抗原檢測、糞便彈性蛋白酶檢測、顯微鏡檢查和其他檢測)、產品類型(儀器和試劑)、模式(實驗室檢測和即時檢測)、應用(腸躁症、潰瘍性結腸炎、克隆氏症、癌症、腹瀉、痔瘡等)、最終
|
覆蓋國家
|
日本、中國、韓國、印度、新加坡、泰國、印尼、馬來西亞、菲律賓、澳洲、紐西蘭、越南、台灣、汶萊、尼泊爾、孟加拉、斯里蘭卡、巴基斯坦、香港、馬爾地夫、蒙古、柬埔寨、寮國、亞太其他地區
|
涵蓋的市場參與者
|
雅培(美國)、Meridian Bioscience Inc.(美國)、BIOMERIEUX(法國)、Beckman Coulter, Inc.(丹納赫子公司)(美國)、DiaSorin SpA(義大利)、潤美(中國)、Molbio Diagnostics Pvt. Ltd.(印度)、Quidel Corporation(中國)、Prians(中國) Diagnostics(積水化學株式會社旗下企業)(美國)、BioVendor Group(捷克共和國)、LifeSign LLC(美國)、Alfa Scientific Designs (US), Inc.、Eiken Chemical Co., Ltd.(日本)、SD Biosensor, INC.(韓國)、Cenogenics Corporation(美國)、美國(中國) Co.(Atech) Co. GmbH(德國)以及北京華根安邦科技有限公司(中國)等
|
報告涵蓋的數據點
|
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。
|
細分分析:
糞便診斷市場分為測試類型、產品類型、模式、應用、最終用戶和分銷管道。
- 根據測試類型,亞太糞便診斷市場分為糞便潛血檢查、糞便生物標記測試、病原體測試、糞便抗原測試、糞便彈性蛋白酶測試、顯微鏡測試和其他測試。
預計到 2023 年,病原體檢測領域將主導糞便診斷市場。
2023 年,由於城市化進程加快和西方食品的普及,病原體檢測領域預計將佔據市場主導地位,從而導致整個地區的胃腸道感染增加。病原體檢測領域預計將在 2023-2030 年間達到 7.7% 的最高複合年增長率。
- 根據產品類型,亞太糞便診斷市場分為儀器和試劑。儀器部分細分為顯微鏡、顯微鏡載玻片、顯微鏡蓋玻片、糞便分析儀、電子秤等。 2023 年,由於診斷多種胃腸道疾病的糞便生物標記研究需求不斷增長,試劑領域預計將佔據市場主導地位
- 根據模式,亞太糞便診斷市場分為實驗室檢測和即時診斷。 2023 年,由於潰瘍性結腸炎等胃腸道相關疾病的增加,以及由此引發的慢性疾病等嚴重健康問題,預計實驗室檢測領域將佔據市場主導地位
- 根據應用,亞太糞便診斷市場分為腸躁症、潰瘍性結腸炎、克隆氏症、癌症、腹瀉、痔瘡等。 2023 年,由於不健康飲食習慣的增加,腹瀉類藥物預計將佔據市場主導地位
- 根據最終用戶,亞太糞便診斷市場細分為醫院、診斷中心、實驗室、專科診所、學術機構和研究中心等。 2023 年,醫院部門預計將佔據市場主導地位,因為醫院擁有先進的儀器、專業的外科醫生以及透過糞便檢測進行診斷的設施
- 根據分銷管道,亞太糞便診斷市場分為直接招標、零售、線上銷售和其他。
到 2023 年,腹瀉領域預計將佔據糞便診斷市場應用領域的最大份額。
到 2023 年,由於不健康飲食習慣的增加,腹瀉類疾病預計將佔據市場主導地位。預計 2023 年至 2030 年腹瀉領域的複合年增長率為 8.2%。
主要參與者
Data Bridge Market Research 認為以下公司是糞便診斷 市場的主要參與者,它們是雅培 (美國)、Meridian Bioscience Inc. (美國)、BIOMERIEUX (法國)、Beckman Coulter, Inc. (Danaher 的子公司) (美國)、DiaSorin SpA (意大利)、潤美 (中國)、Molbio Diagnostics Pvt。 Ltd.(印度)、Quidel Corporation(美國)、Prenetics Limited(共和國)、LifeSign LLC(美國)、Alfa Scientific Designs (US), Inc.、Eiken Chemical Co., Ltd.(日本)、SD Biosensor, INC.(韓國)、Cenogenics Corporation(美國)、TransGen Biotech Co., Ltda(中國)、Advion)、Kibion) GmbH(德國)和北京華根安邦科技有限公司(中國)等。
市場發展
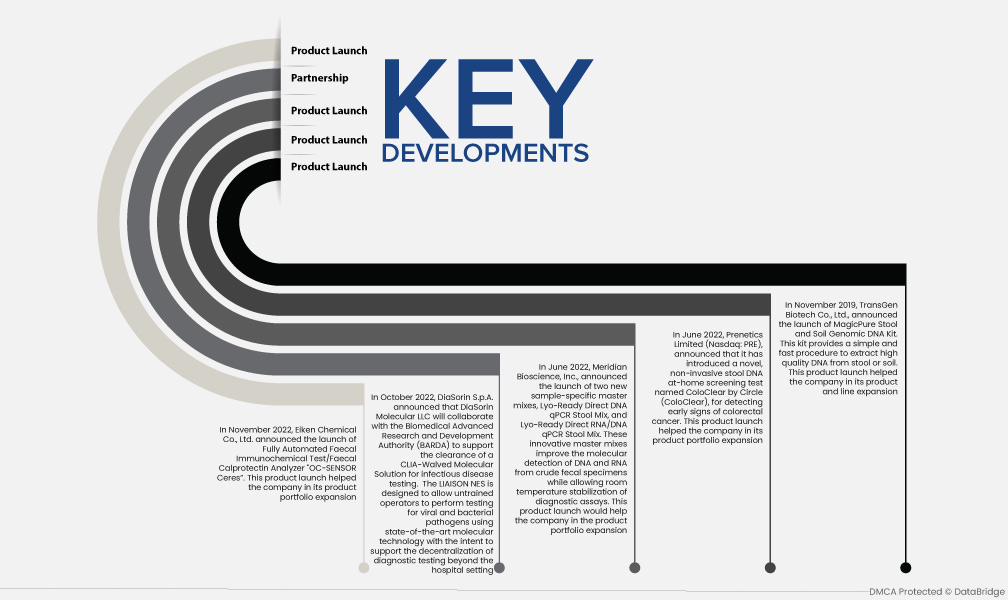
- 2022年11月,榮研化學株式會社宣布推出全自動糞便免疫化學檢測/糞鈣衛蛋白分析儀「OC-SENSOR Ceres」。該產品的推出有助於公司擴大其產品組合
- 2022 年 10 月,DiaSorin SpA 宣布 DiaSorin Molecular LLC 與生物醫學高級研究與發展局 (BARDA) 合作,支持批准用於傳染病檢測的 CLIA-Walved 分子解決方案。 LIAISON NES 旨在允許未經培訓的操作員使用最先進的分子技術對病毒和細菌病原體進行檢測,旨在支援將診斷檢測分散到醫院環境之外
- 2022 年 6 月,Meridian Bioscience, Inc. 宣布推出兩種新的樣本專用主混合物,Lyo-Read Direct DNA qPCR Stool Mix 和 Lyo-Read Direct RNA/DNA qPCR Stool Mix。這些創新的主混合物可改善粗糞便樣本中的 DNA 和 RNA 分子檢測,同時讓診斷檢測在室溫下保持穩定。該產品的推出將有助於公司擴大其產品組合
- 2022 年 6 月,Prenetics Limited (Nasdaq: PRE) 宣布推出一種名為 ColoClear by Circle 的新型非侵入性糞便 DNA 家用篩檢測試(ColoClear),用於檢測大腸直腸癌的早期跡象。該產品的推出有助於公司擴大其產品組合
- 2019年11月,全式金生物科技股份有限公司宣布推出MagicPure糞便及土壤基因組DNA檢測試劑盒。該試劑盒提供了一種從糞便或土壤中提取高品質 DNA 的簡單、快速的方法。該產品的推出有助於公司的產品和生產線的擴展
區域分析
從地理上看,糞便診斷市場報告涵蓋的國家有日本、中國、韓國、印度、新加坡、泰國、印尼、馬來西亞、菲律賓、澳洲、紐西蘭、越南、台灣、汶萊、尼泊爾、孟加拉、斯里蘭卡、巴基斯坦、香港、馬爾地夫、蒙古、柬埔寨、寮國和亞太其他地區。
根據數據橋市場研究分析
2023年至2030年預測期內,中國是糞便診斷市場的主導地區
預計到2023年,中國將以8.69%的市佔率主導亞太糞便診斷市場。這是由於胃腸道疾病病例增加以及幾家主要市場參與者推出了用於糞便診斷的新型檢測試劑盒。
有關糞便診斷市場的更多詳細信息,請點擊此處 - https://www.databridgemarketresearch.com/reports/asia-pacific-stool-diagnostic-market