North America Balloon Catheter Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
1.83 Billion
USD
2.97 Billion
2024
2032
USD
1.83 Billion
USD
2.97 Billion
2024
2032
| 2025 –2032 | |
| USD 1.83 Billion | |
| USD 2.97 Billion | |
|
|
|
|
Сегментация рынка баллонных катетеров в Северной Америке по типу (баллонные катетеры PTCA, баллонные катетеры CTO и микрокатетеры), типу продукта (обычный баллонный катетер, баллонный катетер с лекарственным покрытием, режущий баллонный катетер, баллонный катетер со стент- графтом и надрезающий баллонный катетер), платформе доставки (баллонный катетер быстрого обмена (RX) / монорельсовый, баллонный катетер с проводником (OTW) и баллонный катетер с фиксированной проволокой (FW)), соответствию (несоответствующий, полусоответствующий и соответствующий), материал баллона ( нейлонполиэтилентерефталат (ПЭТ), полиэтилен (ПЭ), силикон, полиолефиновый сополимер и другие), тип баллона (баллоны высокого давления и эластомерные баллоны), применение (ишемическая болезнь сердца, заболевание периферических артерий и другие), конечный пользователь (больницы, Специализированные центры, центры амбулаторной хирургии и другие), каналы сбыта (прямые тендеры, дистрибуция третьими лицами и другие) – тенденции отрасли и прогноз до 2032 года
Размер рынка баллонных катетеров в Северной Америке
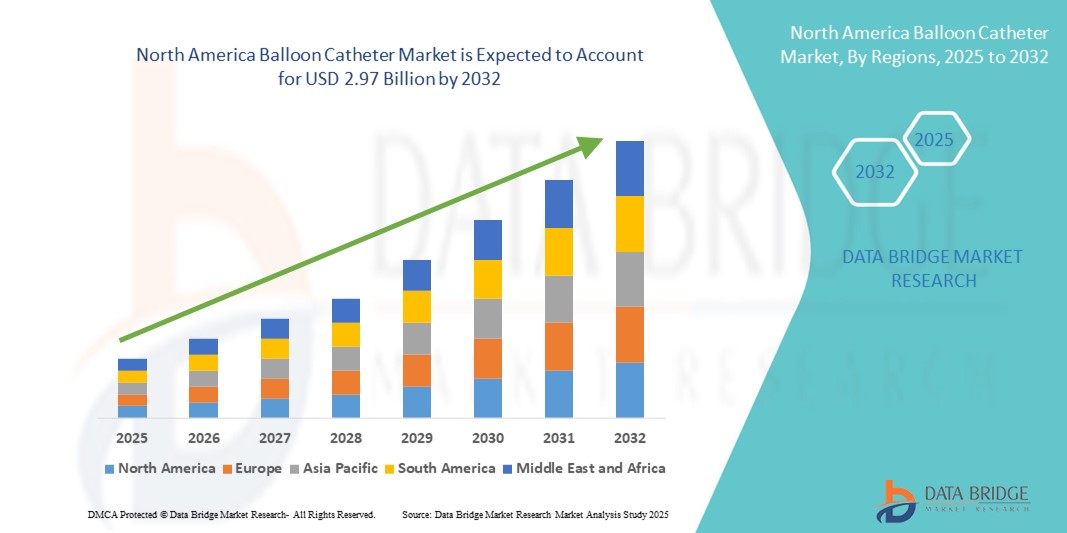
- Объем рынка баллонных катетеров в Северной Америке в 2024 году оценивался в 1,83 млрд долларов США , а к 2032 году , как ожидается, он достигнет 2,97 млрд долларов США при среднегодовом темпе роста 6,20% в течение прогнозируемого периода.
- Рост рынка во многом обусловлен увеличением числа сердечно-сосудистых заболеваний, ростом спроса на малоинвазивные процедуры и постоянными достижениями в области катетерных технологий, что способствует широкому внедрению баллонных катетеров в регионе Северной Америки.
- Кроме того, рост расходов на здравоохранение, улучшение инфраструктуры больниц и растущее внимание к ранней диагностике и интервенционному лечению ускоряют внедрение решений с использованием баллонных катетеров в Северной Америке, тем самым значительно стимулируя рост отрасли.
Анализ рынка баллонных катетеров в Северной Америке
- Баллонные катетеры становятся все более важным компонентом интервенционной кардиологии и радиологии в Северной Америке, особенно в больницах и специализированных клиниках, из-за растущей распространенности сердечно-сосудистых заболеваний и заболеваний периферических артерий, достижений в области минимально инвазивных процедур и повышения осведомленности о раннем вмешательстве.
- Растущий спрос на процедуры с использованием баллонного катетера обусловлен, прежде всего, растущим бременем заболеваний, связанных с образом жизни, таких как диабет и гипертония, государственными инвестициями в инфраструктуру здравоохранения и растущим внедрением процедур ангиопластики в развивающихся экономиках региона.
- США доминировали на североамериканском рынке баллонных катетеров с наибольшей долей выручки в 39,6% в 2024 году, что обусловлено развитой инфраструктурой здравоохранения, большими объемами процедур в интервенционной кардиологии и значительными инвестициями в исследования и разработки в области минимально инвазивной сосудистой терапии.
- Ожидается, что Канада станет страной с самыми быстрыми темпами роста на североамериканском рынке баллонных катетеров в течение прогнозируемого периода, чему будут способствовать повышение осведомленности о сердечно-сосудистых заболеваниях, старение населения и правительственные инициативы, направленные на улучшение ранней диагностики и доступа к эндоваскулярным процедурам.
- Сегмент баллонов высокого давления доминировал на североамериканском рынке баллонных катетеров с долей рынка 61,8% в 2024 году. Он предпочтителен для использования в сильно кальцинированных поражениях, где повышенная радиальная прочность и контролируемое надувание имеют решающее значение для успешного расширения поражения и развертывания стента.
Область применения отчета и сегментация рынка баллонных катетеров в Северной Америке
|
Атрибуты |
Ключевые данные о рынке баллонных катетеров в Северной Америке |
|
Охваченные сегменты |
|
|
Охваченные страны |
Северная Америка
|
|
Ключевые игроки рынка |
|
|
Рыночные возможности |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, отчеты о рынке, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, анализ цен, анализ доли бренда, опрос потребителей, демографический анализ, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья/расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка баллонных катетеров в Северной Америке
Технологические достижения и бесшовная системная интеграция
- Важной и набирающей обороты тенденцией на североамериканском рынке баллонных катетеров является растущая интеграция передовых технологий, повышающих эффективность процедур и улучшающих результаты лечения. Инновации в конструкции катетеров, материаловедении и совместимости с визуализацией в реальном времени значительно повышают точность и безопасность процедур баллонной ангиопластики как при коронарных, так и при периферических вмешательствах.
- Например, современные баллонные катетеры с лекарственным покрытием теперь адаптируются для сложных поражений и сосудов меньшего размера, что позволяет интервенционным кардиологам проводить целенаправленную терапию с минимальным риском рестеноза. Аналогичным образом, баллонные катетеры с надрезами и режущими элементами оптимизируются для кальцинированных поражений, обеспечивая более высокую эффективность процедур в сложных случаях.
- Эти достижения также поддерживаются интеллектуальными инструментами планирования процедур и диагностической обратной связью в режиме реального времени от таких систем визуализации, как ВСУЗИ (внутрисосудистое ультразвуковое исследование) и ОКТ (оптическая когерентная томография), которые позволяют с большей точностью управлять установкой катетера. Внедрение таких интегрированных интервенционных комплексов стремительно растёт в больницах третьего уровня и специализированных кардиологических центрах по всей Северной Америке.
- Более того, полная совместимость баллонных катетеров с новыми роботизированными интервенционными платформами способствует установлению нового стандарта в малоинвазивной сосудистой хирургии. Эти роботизированные системы обеспечивают точную навигацию катетеров по извилистым сосудистым структурам, снижая утомляемость оператора и лучевую нагрузку, а также улучшая контроль над процедурой.
- Эта тенденция к большей интероперабельности, автоматизации и персонализации меняет ожидания врачей и стандарты ухода за пациентами в регионе. В связи с этим производители разрабатывают баллонные катетеры нового поколения с такими функциями, как технология измерения давления, управляемость и настраиваемые профили раздувания, которые могут адаптироваться к характеристикам сосудов в режиме реального времени.
- Спрос на такие передовые, интуитивно понятные и интегрированные решения для баллонной катетеризации стремительно растет в странах Северной Америки, что обусловлено растущим бременем сердечно-сосудистых заболеваний, увеличением инвестиций в модернизацию катетеризационных лабораторий и большим акцентом на результатах, основанных на ценностях, в системах здравоохранения.
Динамика рынка баллонных катетеров в Северной Америке
Водитель
Растущая потребность в связи с ростом числа сердечно-сосудистых заболеваний и технологическим прогрессом
- Растущая распространенность сердечно-сосудистых заболеваний (ССЗ) в Северной Америке в сочетании со старением населения и нездоровым образом жизни существенно повышает спрос на процедуры с использованием баллонного катетера, особенно при вмешательствах на коронарных и периферических артериях.
- Например, в марте 2024 года Министерство здравоохранения Саудовской Аравии запустило общенациональную инициативу по скринингу сердечно-сосудистых заболеваний, направленную на раннее выявление артериальной закупорки у групп высокого риска, что привело к увеличению спроса на современные катетерные вмешательства в государственных больницах. Ожидается, что такие стратегии, реализуемые под руководством правительства, будут способствовать росту индустрии баллонных катетеров в Северной Америке в прогнозируемый период.
- По мере роста осведомленности о малоинвазивных методах лечения как пациенты, так и медицинские работники все чаще выбирают процедуры с использованием баллонного катетера благодаря сокращенному времени восстановления, минимальной хирургической травме и высоким показателям успешности процедур. Этот сдвиг особенно заметен в городских медицинских учреждениях, где лаборатории катетеризации быстро расширяются.
- Более того, технологические достижения, такие как баллоны с лекарственным покрытием, баллоны с насечкой и баллонные катетеры высокого давления, интегрируются в протоколы интервенционной кардиологии, обеспечивая лучшую подготовку поражений и лучшие результаты после дилатации. Эти инновации позволяют проводить лечение сложных поражений с большей точностью и безопасностью.
- Растущий спрос на экономичные и высокопроизводительные решения, а также рост числа лабораторий катетеризации в таких странах, как США, Мексика и Канада, способствуют внедрению баллонных катетеров. Благоприятная политика возмещения расходов и инициативы в области медицинского туризма также способствуют расширению рынка.
Сдержанность/Вызов
Ограниченный доступ к развитой инфраструктуре и высокие процессуальные издержки
- Доступность современных катетеризационных лабораторий и квалифицированных интервенционных кардиологов остается неравномерной в Северной Америке, особенно в регионах с низким уровнем дохода и сельской местности, что ограничивает доступ к процедурам баллонной катетеризации для значительной части населения.
- Например, страны Африки к югу от Сахары часто сталкиваются с задержками в импорте высокотехнологичных медицинских приборов из-за узких мест в нормативно-правовой базе и проблем с инфраструктурой, что влияет на своевременное проведение процедур с использованием баллонного катетера.
- Высокая стоимость баллонных катетеров с лекарственным покрытием и насечкой также является препятствием для их внедрения в больницах с ограниченным бюджетом и государственных учреждениях здравоохранения. Несмотря на то, что эти технологии обеспечивают превосходные результаты, их доступность и доступность остаются серьёзными препятствиями.
- Кроме того, поддержание стерильных цепочек поставок, обучение работе с устройствами и инфраструктура постпроцедурного мониторинга требуют значительных инвестиций, что может удерживать небольшие больницы и специализированные клиники от внедрения таких систем.
- Преодоление этих проблем посредством регионального производства, местных партнерских отношений с дистрибьюторами, инвестиций в программы обучения в области интервенционной кардиологии и оптимизации нормативно-правовой базы будет иметь решающее значение для обеспечения равноправного доступа и долгосрочного роста рынка баллонных катетеров в Северной Америке.
Рынок баллонных катетеров в Северной Америке
Рынок сегментирован по типу, типу продукта, платформе доставки, соответствию нормативным требованиям, материалу баллона, типу баллона, области применения, конечному пользователю и каналу сбыта.
- По типу
По типу рынок баллонных катетеров в Северной Америке сегментируется на баллонные катетеры для транскатетерной коронарной ангиопластики (ЧТКА), баллонные катетеры для хронической коронарной ангиопластики (ХТО) и микрокатетеры. Сегмент баллонных катетеров для ЧТКА доминировал на рынке, обеспечив наибольшую долю выручки в 54,2% в 2024 году, что обусловлено их широким применением при лечении ишемической болезни сердца и ростом числа чрескожных коронарных вмешательств в регионе.
Ожидается, что сегмент баллонных катетеров CTO продемонстрирует самые высокие темпы роста — 20,6% в период с 2025 по 2032 год, что будет обусловлено их растущим применением при лечении хронических полных окклюзий при сложных сердечно-сосудистых заболеваниях.
- По типу продукта
По типу продукции рынок сегментирован на стандартные баллонные катетеры, баллонные катетеры с лекарственным покрытием, режущие баллонные катетеры, баллонные катетеры для стент-графта и надрезающие баллонные катетеры. Сегмент стандартных баллонных катетеров занимал наибольшую долю рынка в 38,9% в 2024 году благодаря их повсеместному применению при коронарных и периферических вмешательствах, а также широкой доступности.
Прогнозируется, что сегмент баллонных катетеров с лекарственным покрытием будет расти самыми быстрыми темпами в год на уровне 21,3% в период с 2025 по 2032 год, что будет обусловлено более широким применением для профилактики рестеноза и в случаях, когда стенты не подходят.
- По платформе доставки
По типу платформы доставки рынок сегментирован на баллонные катетеры Rapid Exchange (RX)/Monorail, баллонные катетеры Over-The-Wire (OTW) и баллонные катетеры Fixed Wire (FW). Сегмент Rapid Exchange (RX) доминировал на рынке с долей выручки 51,4% в 2024 году, обладая преимуществом за скорость проведения процедуры и возможность использования одним оператором в интервенционной кардиологии.
Ожидается, что сегмент Over-The-Wire (OTW) будет расти самыми быстрыми темпами среднегодового темпа роста на уровне 18,4% в период с 2025 по 2032 год благодаря его превосходной проходимости и управляемости в сложных и извилистых условиях.
- По соблюдению
По степени соответствия рынок сегментируется на некомплаентные, полукомплаентные и комплаентные баллоны. Сегмент полукомплаентных баллонов лидировал с наибольшей долей рынка в 46,8% в 2024 году благодаря балансу гибкости и устойчивости к высокому давлению, что делает их идеальными для различных типов поражений.
Прогнозируется, что сегмент несоответствующих требованиям имплантатов будет расти среднегодовыми темпами на 17,9% в период с 2025 по 2032 год в связи с их полезностью в процедурах постдилатации и оптимизации внутристентового пространства.
- По материалу шара
В зависимости от материала, из которого изготовлены баллоны, рынок сегментируется на нейлон, полиэтилентерефталат (ПЭТ), полиэтилен (ПЭ), силикон, полиолефиновый сополимер и другие. Наибольшую долю рынка (42,3%) в 2024 году занимал сегмент ПЭТ, ценимый за высокое разрывное давление и отсутствие требований к соблюдению нормативных требований.
Ожидается, что баллоны на основе силикона будут расти самыми быстрыми среднегодовыми темпами на уровне 19,2% в период с 2025 по 2032 год, что обусловлено их применением при нейроваскулярных и педиатрических вмешательствах.
- По типу воздушного шара
В зависимости от типа баллона рынок сегментирован на баллоны высокого давления и эластомерные баллоны. Баллоны высокого давления доминировали на рынке с долей 61,8% в 2024 году и предпочитали использовать их при сильно кальцинированных поражениях.
Прогнозируется, что объемы производства эластомерных баллонов будут расти среднегодовыми темпами на 18,8% в период с 2025 по 2032 год. Они будут использоваться в приложениях, требующих более низкого давления при высокой гибкости.
- По применению
По области применения рынок сегментируется на ишемическую болезнь сердца, заболевания периферических артерий и другие. Наибольшая доля выручки в 2024 году пришлась на сегмент ишемической болезни сердца (ИБС) — 59,4%, что обусловлено высокой заболеваемостью сердечно-сосудистыми заболеваниями и ростом объёма процедур.
Прогнозируется, что заболеваемость периферическими артериями (ЗПА) будет расти самыми быстрыми среднегодовыми темпами на 20,1% в период с 2025 по 2032 год, что объясняется ростом распространенности диабета и сосудистых заболеваний, связанных с курением.
- Конечным пользователем
По типу конечного потребителя рынок сегментирован на больницы, специализированные центры, центры амбулаторной хирургии и другие. Больницы заняли лидирующие позиции, обеспечив наибольшую долю выручки в 64,8% в 2024 году благодаря развитой инфраструктуре и большому объёму процедур.
Ожидается, что в центрах амбулаторной хирургии будет зарегистрирован самый быстрый среднегодовой темп роста — 21,6%, что отражает переход к малоинвазивным амбулаторным методам лечения.
- По каналу распространения
По каналу сбыта рынок сегментируется на сегменты прямых тендеров, дистрибуции через третьих лиц и прочие. В 2024 году на сегмент прямых тендеров приходилось 58,1% доли рынка, что обусловлено оптовыми институциональными закупками и централизованными закупками.
Ожидается, что в сегменте дистрибуции третьими лицами будут наблюдаться самые быстрые темпы среднегодового роста в период с 2025 по 2032 год, чему будет способствовать расширение сетей частных больниц и дистрибьюторских сетей.
Региональный анализ рынка баллонных катетеров в Северной Америке
- Рынок баллонных катетеров в Северной Америке демонстрирует устойчивый рост, обусловленный растущим бременем сердечно-сосудистых заболеваний, увеличением численности пожилых людей и значительным переходом к малоинвазивным процедурам при коронарных и периферических вмешательствах.
- Хорошо развитая инфраструктура здравоохранения в регионе, благоприятная среда возмещения расходов и раннее внедрение передовых интервенционных технологий являются ключевыми факторами, ускоряющими расширение рынка.
- Высокие объемы процедур в больницах и амбулаторных хирургических центрах в сочетании с наличием передовых устройств, таких как катетеры с лекарственным покрытием, оценочные катетеры и баллонные катетеры высокого давления, укрепляют лидерство региона на мировом рынке.
Обзор рынка баллонных катетеров в США
Рынок баллонных катетеров в США является крупнейшим и наиболее зрелым в Северной Америке, что обусловлено высоким уровнем сердечно-сосудистых заболеваний, развитой инфраструктурой здравоохранения и постоянными инновациями в области минимально инвазивных технологий. Присутствие ведущих производителей медицинского оборудования и широкое внедрение передовых методов интервенционной кардиологии стимулируют использование баллонных катетеров с лекарственным покрытием, оценочных и высокого давления. США доминировали на североамериканском рынке баллонных катетеров с наибольшей долей выручки в 39,6% в 2024 году благодаря значительным объемам процедур, благоприятной политике возмещения расходов и высокой концентрации кардиологических центров. Кроме того, государственные и частные инвестиции в исследования и клинические испытания продолжают способствовать расширению рынка, особенно в области лечения сложных ишемических заболеваний сердца и периферических артерий.
Обзор рынка баллонных катетеров в Канаде
Ожидается, что рынок баллонных катетеров в Канаде станет самым быстрорастущим в Северной Америке в течение прогнозируемого периода, чему способствуют старение населения, рост распространенности факторов риска сердечно-сосудистых заболеваний и расширение возможностей интервенционной кардиологии в разных провинциях. Увеличение финансирования государственного здравоохранения, а также стратегические закупки больницами современных систем баллонных катетеров, способствуют их внедрению. Акцент Канады на ранней диагностике и малоинвазивной терапии, особенно в рамках всеобщего здравоохранения, приводит к более широкому использованию баллонных катетеров, соответствующих требованиям, не соответствующих требованиям и покрытых лекарственными препаратами. Партнерство между академическими учреждениями и производителями устройств дополнительно ускоряет инновации и внедрение катетерных решений нового поколения в области кардиологии и сосудистой хирургии.
Обзор рынка баллонных катетеров в Мексике
Рынок баллонных катетеров в Мексике набирает обороты благодаря улучшению доступа к специализированной кардиологической помощи, росту инвестиций в частное здравоохранение и повышению осведомленности о методах интервенционного лечения сердечно-сосудистых заболеваний. Урбанизация и рост распространенности ожирения, диабета и гипертонии повышают спрос на малоинвазивные сердечно-сосудистые процедуры. Больницы в крупных городах, таких как Мехико, Гвадалахара и Монтеррей, все чаще используют баллонные катетеры для лечения ишемической болезни сердца и заболеваний периферических артерий. Более того, близость Мексики к США способствует импорту передовых медицинских технологий, обеспечивая доступность различных типов баллонных катетеров, включая катетеры для чрескожной транслюминальной коронарной ангиопластики (ЧТКА), скоринговые и с лекарственным покрытием.
Доля рынка баллонных катетеров в Северной Америке
Лидерами североамериканской отрасли баллонных катетеров являются, в первую очередь, хорошо зарекомендовавшие себя компании, в том числе:
- Бостонская научная корпорация (США)
- Medtronic (Ирландия)
- Эбботт (США)
- Корпорация Терумо (Япония)
- BD (США)
- Б. Браун СЕ (Германия)
- Конинклийке Philips NV (Нидерланды)
- Teleflex Incorporated (США)
- Кук (США)
- Lepu Medical Technology (Beijing) Co., Ltd. (Китай)
- KANEKA CORPORATION (Япония)
- MicroPort Scientific Corporation (Китай)
- Acrostak Int. Distr. Sàrl (Switzerland)
- Биотроник (Германия)
- Альвимедика (Турция)
- SMT (Индия)
- БАЛТОН (Польша)
- APR Medtech Ltd (Англия)
- Advin Health Care (Индия)
Последние события на рынке баллонных катетеров в Северной Америке
- В июне 2025 года в Берлине состоялся 8-й Всемирный конгресс по таргетной фаговой терапии , собравший более 75 экспертов из 27 стран. Мероприятие было посвящено внедрению исследований фагов в клиническую практику, включая такие темы, как генно-инженерные фаги, нормативно-правовая база, производство в соответствии с требованиями GMP и клинические примеры инфекций с множественной лекарственной устойчивостью (МЛУ).
- В июле 2025 года компания Cellexus (Великобритания) подчеркнула реальный эффект фаговой терапии, отметив её потенциал для снижения расходов на здравоохранение и улучшения результатов лечения в Европе. В статье описывалось, как фаговая терапия способствует масштабным усилиям по борьбе с устойчивостью к противомикробным препаратам (УПП).
- В ноябре 2023 года компания Boston Scientific завершила сделку по приобретению компании Relevant Medsystems, добавив систему внутрикостной абляции нервов Intracept в свой портфель решений для лечения хронической боли. Сделка, стоимость которой составила 850 миллионов долларов США (авансом плюс условные платежи), расширяет доступ к лечению вертеброгенной боли благодаря национальному страховому покрытию, что принесло пользу более чем 150 миллионам жизней.
- В апреле 2023 года компания Abbott завершила приобретение компании Cardiovascular Systems, Inc., известной своей инновационной системой атерэктомии, используемой для лечения сосудистых заболеваний. Система CSI, предназначенная для подготовки сосудов к ангиопластике или стентированию, вошла в портфель продуктов Abbott, связанных с сосудистыми заболеваниями. В тот же день акции CSI прекратили торговаться на Nasdaq.
- В августе 2022 года компания Boston Scientific объявила о приобретении компании Obsidio, Inc., разработавшей технологию GEM для эмболизации периферических сосудов. Полутвердый материал GEM, недавно одобренный FDA, упрощает процедуры эмболизации благодаря своим уникальным гелеобразным свойствам. Ожидалось, что эта сделка не окажет существенного влияния на прибыль Boston Scientific в 2022 году.
- В феврале 2022 года компания Medtronic получила одобрение FDA на катетеры Freezor и Freezor Xtra для лечения атриовентрикулярной узловой реципрокной тахикардии (АВРНТ) у детей. Эта криоаблационная терапия эффективна у более чем 140 000 пациентов по всему миру и направлена на устранение нарушений сердечного ритма у детей, помогая предотвратить опасные для жизни осложнения и поддерживать нормальную работу сердца.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.