Middle East And Africa Thyroid Ablation Devices Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
60.51 Billion
USD
126.90 Billion
2025
2033
USD
60.51 Billion
USD
126.90 Billion
2025
2033
| 2026 –2033 | |
| USD 60.51 Billion | |
| USD 126.90 Billion | |
|
|
|
|
Рынок устройств для абляции щитовидной железы на Ближнем Востоке и в Африке по заболеваниям (доброкачественные узлы щитовидной железы, рак щитовидной железы), типу продукта (термические устройства, нетермические устройства), модальности (стационарные, автономные, портативные), типу (радиочастотная абляция, лазерная или световая абляция, ультразвуковая абляция, криоабляция, электрическая абляция, микроволновая абляция, гидротермальная абляция и другие), конечный пользователь (больницы и хирургические центры, онкологические центры, клиники, общественное здравоохранение и другие), канал распространения (прямые продажи, сторонний дистрибьютор), страна (Саудовская Аравия, Южная Африка, ОАЭ, Израиль, Египет и остальные страны Ближнего Востока и Африки) Тенденции отрасли и прогноз до 2028 года
Анализ рынка и аналитика: рынок устройств для абляции щитовидной железы на Ближнем Востоке и в Африке
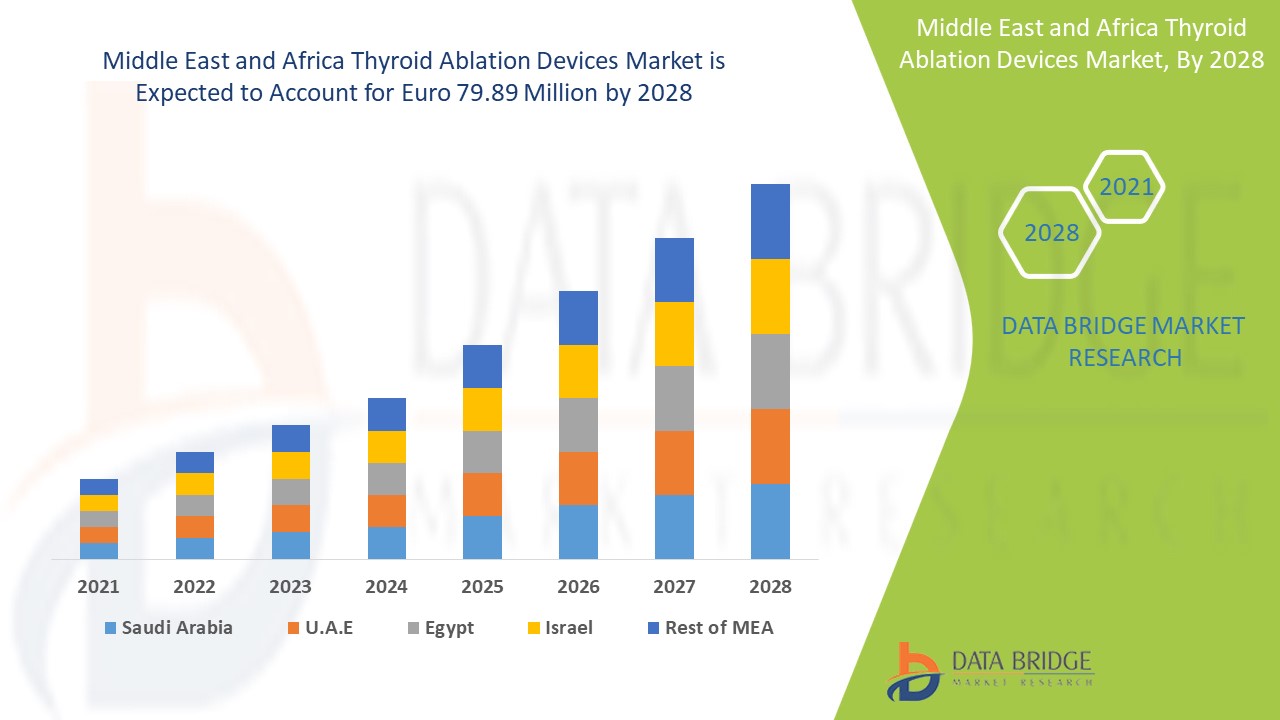
Ожидается, что рынок устройств для абляции щитовидной железы будет расти в прогнозируемый период с 2021 по 2028 год. По данным Data Bridge Market Research, рынок растет со среднегодовым темпом роста 9,7% в прогнозируемый период с 2021 по 2028 год и, как ожидается, достигнет 79,89 млн евро к 2028 году. Рост распространенности узлов щитовидной железы и рака увеличивает спрос на процедуры абляции щитовидной железы на рынке устройств для абляции щитовидной железы.
Абляция опухоли — это минимально инвазивная процедура для лечения опухолей печени, почек, костей и легких, а также других мягкотканных желез. Метод абляции используется для лечения узлов щитовидной железы и рака. Узел щитовидной железы — это комок клеток щитовидной железы, который аномально развился в щитовидной железе. Нормальная ткань щитовидной железы часто может начать развиваться, что приводит к развитию одного или нескольких узлов.
Прогресс в методах абляции позволяет использовать на рынке больше новых процедур и продуктов, что увеличивает рост рынка устройств для абляции щитовидной железы. Высокая стоимость процедур абляции узлов щитовидной железы ограничивает пациентов в выборе различных типов процедур абляции, что препятствует росту рынка устройств для абляции щитовидной железы. Увеличение гериатрического населения и более высокий риск образования узлов щитовидной железы у пожилых людей будут выступать в качестве возможности для рынка устройств для абляции щитовидной железы. Нехватка квалифицированного персонала может привести к нарушению процедуры, поэтому является проблемой для рынка устройств для абляции щитовидной железы.
Отчет о рынке устройств для абляции щитовидной железы на Ближнем Востоке и в Африке содержит подробную информацию о доле рынка, новых разработках и анализе линейки продукции, влиянии внутренних и локальных игроков рынка, анализирует возможности с точки зрения новых источников дохода, изменений в рыночных правилах, одобрений продуктов, стратегических решений, запусков продуктов, географических расширений и технологических инноваций на рынке. Чтобы понять анализ и рыночный сценарий, свяжитесь с нами для получения аналитического обзора, наша команда поможет вам создать решение по влиянию на доход для достижения желаемой цели.
Объем и размер рынка устройств для абляции щитовидной железы на Ближнем Востоке и в Африке
Рынок устройств для абляции щитовидной железы подразделяется на шесть заметных сегментов, которые основаны на заболевании, типе продукта, модальности, типе, конечном пользователе и канале сбыта. Рост среди сегментов помогает вам анализировать нишевые карманы роста и стратегии для выхода на рынок и определять ваши основные области применения и разницу в ваших целевых рынках.
- На основе заболевания рынок устройств для абляции щитовидной железы сегментирован на доброкачественные узлы щитовидной железы и рак щитовидной железы. В 2021 году сегмент доброкачественных узлов щитовидной железы является широко распространенным заболеванием, и менее 1% узлов преобразуются в рак щитовидной железы, что увеличивает спрос на продукцию для доброкачественных узлов щитовидной железы, а не для рака щитовидной железы.
- На основе типа продукта рынок устройств для абляции щитовидной железы сегментируется на термические устройства и нетермические устройства. В 2021 году сегмент термических устройств имеет большой ассортимент продукции, доступной на рынке, и простота доступа к этим устройствам является основной причиной закупки термических устройств.
- По типу рынок устройств для абляции щитовидной железы сегментируется на радиочастотную абляцию, лазерную или световую абляцию, ультразвуковую абляцию, электрическую абляцию, криоабляцию, микроволновую абляцию, гидротермальную абляцию и другие. В 2021 году сегмент радиочастотной абляции доминирует на рынке, поскольку это более простая и надежная процедура со стороны медицинских работников, а частота пациентов, проходящих лечение с помощью этой процедуры, соответственно выше по сравнению с другими.
- На основе модальности рынок устройств для абляции щитовидной железы сегментируется на портативные, стационарные и автономные. В 2021 году стационарный сегмент доминирует на рынке, поскольку большее количество продуктов, доступных на рынке, относится к категории стационарных.
- На основе конечного пользователя рынок устройств для абляции щитовидной железы сегментирован на больницы и хирургические центры, онкологические центры, клиники, общественные медицинские учреждения и т. д. В 2021 году сегмент больниц и хирургических центров разделяет крупнейший рынок из-за большого количества пациентов, проходящих процедуры абляции щитовидной железы в больницах, а также поддержки со стороны правительства для обеспечения хорошо оснащенных инфраструктурных разработок и разработок инструментов в больницах.
- На основе канала сбыта рынок устройств для абляции щитовидной железы сегментируется на прямые продажи и сторонних дистрибьюторов. В 2021 году сегмент прямых продаж доминирует на рынке, поскольку является основным источником закупок в больницах и хирургических центрах.
Анализ рынка устройств для абляции щитовидной железы на уровне страны
Проведен анализ рынка устройств для абляции щитовидной железы, а также предоставлена информация о размере рынка по странам, заболеваниям, типу продукта, модальности, типу, конечному пользователю и каналу сбыта, как указано выше.
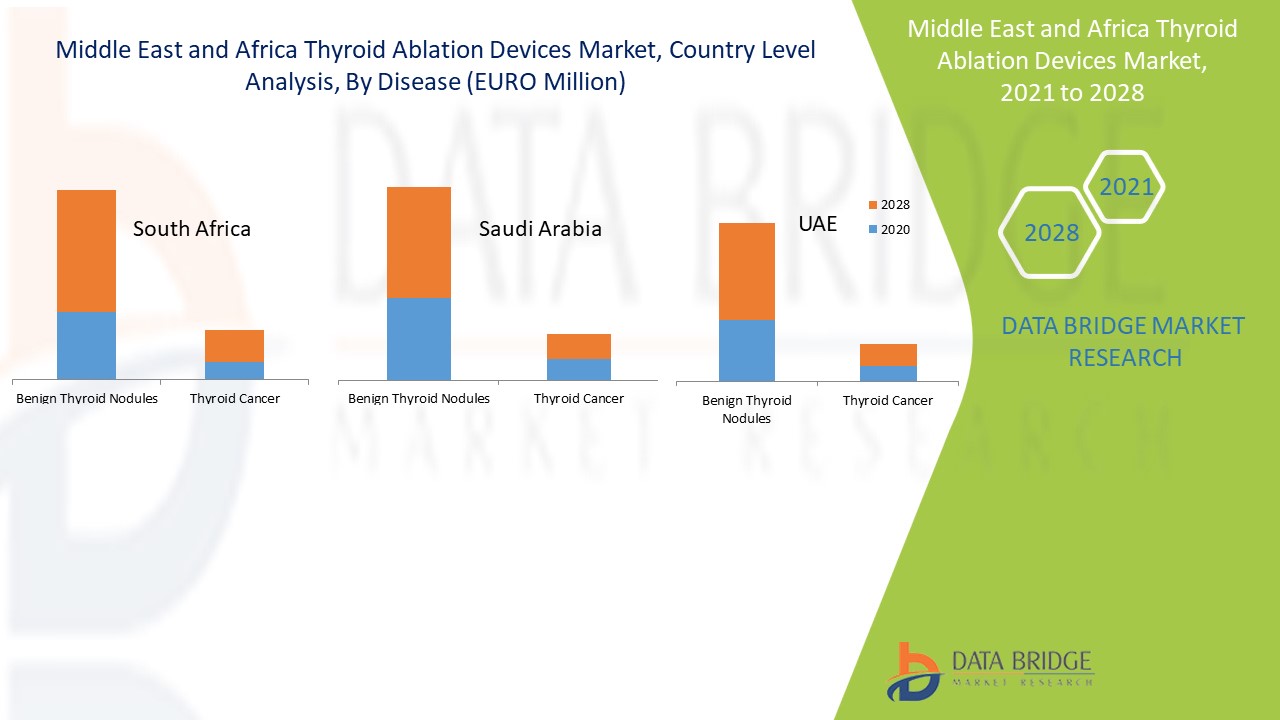
Страны, охваченные рынком устройств для абляции щитовидной железы на Ближнем Востоке и в Африке, включают Саудовскую Аравию, Южную Африку, ОАЭ, Израиль, Египет и остальные страны Ближнего Востока и Африки.
Южная Африка в регионе Ближнего Востока и Африки доминирует на рынке устройств для абляции щитовидной железы благодаря прогрессу в технологии устройств для абляции и наибольшему количеству центров абляции, и было подсчитано, что в Южной Африке проводится около 60 000 процедур катетерной абляции в год. Ожидается, что Саудовская Аравия будет расти со вторым по величине темпом роста в прогнозируемый период с 2021 по 2028 год из-за внедрения технологических достижений и увеличения расходов на здравоохранение. ОАЭ доминируют на рынке устройств для абляции щитовидной железы на Ближнем Востоке и в Африке, занимая третью по величине долю рынка из-за увеличения количества центров абляции для удаления опухолей.
Раздел отчета по странам также содержит отдельные факторы, влияющие на рынок, и изменения в регулировании на внутреннем рынке, которые влияют на текущие и будущие тенденции рынка. Такие данные, как продажи, одобрения FDA, демографические данные страны, нормативные акты и импортно-экспортные тарифы, являются одними из основных указателей, используемых для прогнозирования рыночного сценария для отдельных стран. Кроме того, при предоставлении прогнозного анализа данных по стране учитываются наличие и доступность мировых производителей брендовых и дженериковых препаратов и их проблемы, связанные с большой или малой конкуренцией со стороны местных и отечественных брендов, влияние каналов продаж.
Рост численности гериатрического населения создает новые возможности для игроков на рынке устройств для абляции щитовидной железы
Рынок устройств для абляции щитовидной железы также предоставляет вам подробный анализ рынка для каждой страны, рост в определенной отрасли с продажами устройств для абляции щитовидной железы, влияние прогресса в устройствах для абляции щитовидной железы и изменения в сценариях регулирования с их поддержкой рынка устройств для абляции щитовидной железы. Данные доступны за исторический период с 2011 по 2019 год.
Анализ конкурентной среды и доли рынка устройств для абляции щитовидной железы
Конкурентная среда рынка устройств для абляции щитовидной железы содержит сведения по конкурентам. Включены сведения о компании, финансах компании, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, производственных площадках и объектах, сильных и слабых сторонах компании, запуске продукта, испытаниях продукта, одобрении продукта, патентах, широте и широте продукта, доминировании приложений, жизненно важной кривой технологий. Приведенные выше данные относятся только к фокусу компании на рынке устройств для абляции щитовидной железы на Ближнем Востоке и в Африке.
Некоторые из основных игроков, работающих на рынке устройств для абляции щитовидной железы на Ближнем Востоке и в Африке, включают Medtronic, Biosense Webster, Inc, Boston Scientific Corporation, Olympus Coropration, Smith & Nephew., Koninklijke Philips NV и BIOTRONIK SE & Co. KG, Terumo Europe NV (дочерняя компания Terumo Corporation), Stryker, Merit Medical Systems, IceCure Medical, Biosense Webster, Inc. (дочерняя компания Johnson & Johnson Services, Inc.) и другие. Аналитики DBMR понимают конкурентные преимущества и предоставляют конкурентный анализ для каждого конкурента отдельно.
Многие запуски продуктов, одобрения, партнерства и соглашения также инициируются компаниями по всему миру, что также ускоряет развитие европейского рынка устройств для абляции щитовидной железы.
Например,
- В сентябре 2020 года Smith & Nephew объявили, что подписали партнерство с робототехнической компанией Tharus для пилотирования своей инновационной системы социального дистанцирования Bump. В рамках этого соглашения Tharus модернизирует производственный процесс компании, что поможет поддерживать социальную дистанцию и доставлять людей на работу. Это поможет компании удовлетворить глобальный спрос на продукцию.
Сотрудничество, совместные предприятия и другие стратегии участников рынка расширяют возможности компании на рынке устройств для абляции щитовидной железы, что также дает организациям возможность улучшить свое предложение устройств для абляции щитовидной железы.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.