Фармакогенетическое тестирование в психиатрии/рынке депрессии на Ближнем Востоке и в Африке по типу (тревожность, расстройства настроения, депрессия, биполярные расстройства, психотические расстройства и расстройства пищевого поведения ), типу теста (секвенирование всего генома и тесты на основе хромосомной матрицы), типу пациента (дети, взрослые и пожилые), типу гена (CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT и другие), продуктам (инструментам, расходным материалам, программному обеспечению и услугам), конечному пользователю (больницам и клиникам, диагностическим лабораториям, академическим и исследовательским институтам и другим), каналу сбыта (прямой тендер, сторонняя дистрибуция, больничная аптека и другим) — тенденции отрасли и прогноз 2029
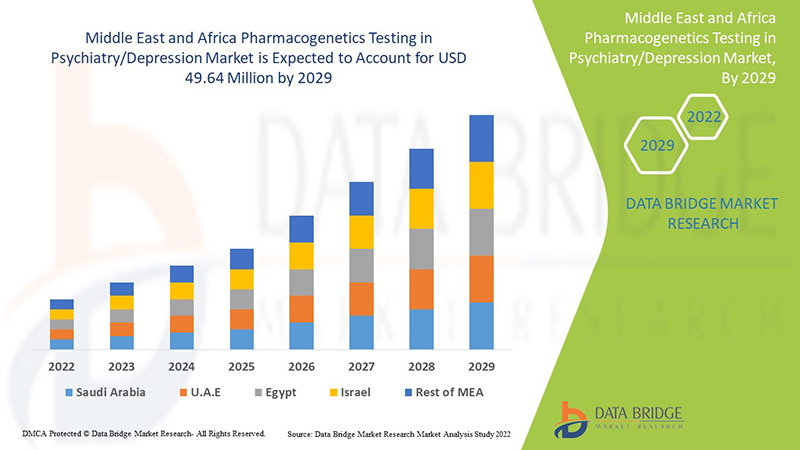
Фармакогенетическое тестирование в психиатрии/анализ рынка депрессии на Ближнем Востоке и в Африке
Фармакогенетическое тестирование помогает медицинским работникам, предоставляя информацию о том, как человек усваивает лекарство. Эта информация может помочь врачам и другим лицам избежать назначения антидепрессантов , которые могут привести к нежелательным результатам. Фармакогеномика показала многообещающие результаты в прогнозировании реакции на антидепрессанты и переносимости при лечении большого депрессивного расстройства (БДР). Фармакогеномика может улучшить клинические результаты, направляя выбор и дозировку антидепрессантов. Растущий сектор биотехнологий и увеличение расходов на здравоохранение ускорили спрос на фармакогенетическое тестирование в психиатрии/депрессии.
Растущая распространенность онкологических заболеваний, новые технологии в лечении депрессии и других психиатрических состояний увеличивают внедрение фармакогенетического тестирования в психиатрии/депрессии устройствах и процедурах, а растущее предпочтение нехирургическим процедурам являются основными движущими силами, которые подстегнули спрос на рынке в прогнозируемый период. Однако высокая стоимость, связанная с тестами, строгое регулирование и недостаточная осведомленность могут, как ожидается, затруднить рост рынка фармакогенетического тестирования в психиатрии/депрессии в прогнозируемый период.
Data Bridge Market Research анализирует, что рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке, как ожидается, достигнет значения 49,64 млн долларов США к 2029 году при среднегодовом темпе роста 7,1% в течение прогнозируемого периода. Тревога составляет самый большой сегмент на рынке из-за растущего уровня депрессии среди населения Ближнего Востока и Африки. Этот отчет о рынке также подробно охватывает анализ цен, патентный анализ и технологические достижения.
|
Отчет Метрика |
Подробности |
|
Прогнозируемый период |
2022-2029 |
|
Базовый год |
2021 |
|
Исторические годы |
2020 |
|
Количественные единицы |
Доход в млн. долл. США, объемы в единицах, цены в долл. США |
|
Охваченные сегменты |
Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке по применению (новые кандидаты на лекарственные препараты, оптимизация и повторное использование лекарственных препаратов, доклиническое тестирование и одобрение, мониторинг лекарственных препаратов, поиск новых мишеней и путей, связанных с заболеваниями, понимание механизмов заболеваний, агрегация и синтез информации, формирование и квалификация гипотез, разработка новых лекарственных препаратов, поиск лекарственных мишеней для старых препаратов и другие), технология (машинное обучение, глубокое обучение, обработка естественного языка и другие), тип препарата (малая молекула и большая молекула), предложение (программное обеспечение и услуги), показание (иммуноонкология, нейродегенеративные заболевания, сердечно-сосудистые заболевания, метаболические заболевания и другие), конечное использование (контрактные исследовательские организации (CRO), фармацевтические и биотехнологические компании, исследовательские центры и академические институты и другие) |
|
Страны, охваченные |
ОАЭ, Израиль, Южная Африка, Египет, Кувейт и остальные страны Ближнего Востока и Африки |
|
Охваченные участники рынка |
Genelex (часть корпорации Invitae), Genewiz (часть Azenta Life Sciences), MD Labs, BiogeniQ, Inc., ONEOME, LLC, Myriad Genetics, Inc., GenXys, Castle Biosciences, Inc., PacBio, QIAGEN, Thermo Fisher Scientific Inc., AB-Biotics, SA, Coriell Life Sciences, Eurofins Scientific, Illumina, Inc., Dynamic DNA Laboratories, STADAPHARM GmbH, Color, cnsdose, Genomind, Inc., Healthspek, myDNA Life Australia Pty Ltd., HudsonAlpha, Sonic Healthcare Limited и другие. |
Фармакогенетическое тестирование в психиатрии/определение рынка депрессии на Ближнем Востоке и в Африке
Фармакогеномное тестирование недавно стало масштабируемым и доступным для управления большим депрессивным расстройством (БДР). Клиницисты все чаще признают фармакогеномное (PGx) тестирование как важный инструмент для управления решениями о приеме лекарств при психиатрических заболеваниях. Широкое внедрение PGx тестирования является движущей силой рынка в прогнозируемый период.
Термины «персонализированная медицина», «стратифицированная медицина» и «точная медицина» являются близкими родственниками фармакогенетики, но это более широкие термины, которые также охватывают дополнительные негенетические факторы. Тем не менее, фармакогенетика является важным компонентом этих областей. Фармакогенетика в первую очередь занимается вариациями ДНК зародышевой линии человека, но также были достигнуты важные недавние успехи в понимании расстройств настроения и психических заболеваний.
Фармакогенетическое тестирование изучает взаимодействие между лекарственным средством и реакцией гена человека и ищет вариацию гена, которая отвечает за влияние на эффект препарата. Тест приобретает большой спрос, поскольку многие исследователи и ученые выявили уникальное взаимодействие между лекарственными средствами и отдельными генами и предоставляют ценные сведения, которые впоследствии могут быть использованы для разработки индивидуальных или персонализированных лекарств.
Динамика рынка фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке
В этом разделе рассматривается понимание движущих сил рынка, преимуществ, возможностей, ограничений и проблем. Все это подробно обсуждается ниже:
Драйверы
- УВЕЛИЧЕНИЕ ЧИСЛА ПАЦИЕНТОВ, СТРАДАЮЩИХ ПСИХИАТРИЧЕСКИМИ И ДЕПРЕССИВНЫМИ РАССТРОЙСТВАМИ
Депрессия является распространенным заболеванием во всем мире, которым страдают, по оценкам, 3,8% населения, в том числе 5,0% взрослых и 5,7% взрослых старше 60 лет. Депрессия может стать серьезным заболеванием от легкой до крайней степени тяжести, заставляя человека сильно страдать и в худшем случае приводя к самоубийству. Хотя доступно более 45 антидепрессантов, неоптимальный ответ представляет собой проблему и считается результатом генетической изменчивости, психиатрии/депрессии. В зависимости от тяжести и характера депрессивных эпизодов с течением времени поставщики медицинских услуг могут предложить психологическую диагностику, такую как поведенческая активация, когнитивно-поведенческая терапия, межличностная психотерапия и/или антидепрессанты, такие как селективные ингибиторы обратного захвата серотонина (СИОЗС) и трициклические антидепрессанты (ТЦА). Для этого вида психического расстройства используются различные препараты.
С ростом распространенности депрессии растет и спрос на фармакогенетическое тестирование, поскольку оно изучает влияние генетических вариантов, намереваясь предоставить индивидуальный диагноз. Ожидается, что рынок будет расти в лесной период.
- РОСТ СПРОСА НА ПЕРСОНАЛИЗИРОВАННУЮ И ТОЧНУЮ МЕДИЦИНУ
Фармакогенетический тест помогает медицинскому работнику выбрать лучшее лекарство для человека, поскольку тест ищет вариант гена, который может отвечать за влияние на эффект препарата.
Медицина начинает становиться персональной, и пациенты постепенно выражают интерес к улучшению результатов и уменьшению побочных эффектов с персонализированными лекарствами. Персонализированная медицина имеет потенциал для адаптации терапии с высоким уровнем безопасности и наилучшим ответом. Эта тенденция во многом обусловлена улучшениями в области секвенирования генома.
Движение к персональному здравоохранению означает изменения в производстве лекарств. Производители переходят от создания малых молекул к сочетанию малых молекул и генной терапии . Спонсоры фокусируются на замене неэффективного крупномасштабного серийного производства инвестициями в новые технологии и производством персонализированных лекарств.
Сдержанность
- Нехватка квалифицированных медицинских и геномных экспертов
Большинство врачей по-прежнему не уверены в фармакогенетическом (PGx) тестировании и последующей интерпретации данных, что указывает на недостаточные знания в этой области. Это подчеркивает необходимость повышения грамотности среди медицинских работников в отношении экспертизы и понимания фармакогенетического (PGx) тестирования.
Недостаточная информированность практикующих врачей о возможностях фармакогенетики, некачественное или недостаточное разъяснение результатов тестов также снижают возможности персонализации технологий для пациентов. Помимо разработки тематических учебных курсов в медицинских вузах, необходимо включение образовательных циклов в системы непрерывного профессионального образования и бесплатное размещение информации для практикующих врачей: академические интернет-порталы, вебинары и т. д. Клинический фармаколог играет важнейшую роль в реализации фармакогенетического тестирования.
Компетентность клинического фармаколога в области фармакогенетики имеет решающее значение: именно он организует применение генотипирования в клинической практике, интерпретирует результаты тестов, информирует врачей о возможностях фармакогенетики для пациентов с конкретными нозологиями, то есть выступает основным связующим звеном между научным миром, системой здравоохранения и практикующими врачами в процессе внедрения фармакогенетики.
Возможность
-
Растущий прогресс в области технологий
Достижения в области фармакогеномики предоставили все больше возможностей для внедрения персонализированной медицины в клиническую практику при психических расстройствах. Персонализированную медицину можно определить как комплексный, перспективный подход к профилактике, диагностике, лечению и мониторингу заболеваний способами, которые позволяют достичь оптимальных индивидуальных решений в области здравоохранения. Более 100 лекарств теперь содержат маркировку Управления по контролю за продуктами и лекарствами США (FDA), связанную с потенциально применимыми фармакогеномными биомаркерами с технологическими достижениями в здравоохранении. Кроме того, разрабатываются новые и передовые методы для содействия фармакогенетическому тестированию при расстройствах, подобных депрессии. Эти тесты используют передовые методы генетического тестирования для получения точных результатов для формирования схемы лечения. Улучшения в технологиях, поддерживающих тесты, улучшили доступность вариантов тестирования, а растущее число ресурсов, помогающих врачам понять, как использовать эту информацию, когда она доступна, делают этот аспект персонализированной или точной медицины реальностью. Таким образом, поставщикам необходимо больше знать о научной и клинической значимости фармакогеномных тестов.
Тесты также помогают установить значимую связь между препаратом и индивидуальным генетическим составом. Это помогает в принятии решения о том, какие препараты следует назначать пациенту для лечения серьезных депрессивных расстройств и других психиатрических состояний.
Испытание
- Строгое государственное регулирование в отношении утверждения новых продуктов и инструментов
Опасения относительно эффективности и безопасности продуктов заставили большинство правительств создать регулирующие агентства и политику для наблюдения за разработкой новых медицинских продуктов или испытаний. Использование этих медицинских продуктов может осуществляться после прохождения строгих нормативных стандартов, которые гарантируют, что продукт безопасен, хорошо изучен и не имеет побочных реакций.
Недавние руководящие принципы и поправка содержат адекватные указания для производителей. Международные правила, такие как пищевые, лекарственные и административные, играют важную роль в новом запуске медицинского продукта или теста на рынок. Таким образом, это может стать серьезным ограничением для рынка. Поэтому строгое государственное регулирование новых продуктов и одобрение инструментов, вероятно, повлияет на рынок.
Влияние COVID-19 на рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке
Вспышка COVID-19 оказала благотворное влияние на расширение индустрии фармакогенетического тестирования. Пандемия оказала негативное влияние на рост рынка фармакогеномики из-за временной остановки исследований в этой области в сочетании с низким притоком пациентов в больницы и диагностические центры. Со второй половины 2020 года, с ростом спроса на исследования определенных препаратов и наборов для тестирования на COVID-19, фармакогеномные практики вошли в моду.
Производители принимают различные стратегические решения, чтобы восстановиться после COVID-19. Игроки проводят многочисленные НИОКР-мероприятия, запускают продукты и создают стратегические партнерства для улучшения технологий и результатов испытаний, задействованных на рынке фармакогенетического тестирования.
Последние события
- В апреле 2022 года Blue Care Network (BCN) запустила программу точной медицины Blue Cross Personalized Medicine, которая использует фармакогеномику или генетическое тестирование для более эффективной персонализации и адаптации медикаментозного лечения для отдельных участников на основе обзора их назначенных лекарств для различных диагнозов, включая поведенческое здоровье, кардиологию, сердечно-сосудистые заболевания и онкологию. OneOme LLC помогла BCN достичь целей программы точной медицины, снизить общую стоимость лечения и улучшить результаты лечения пациентов за счет сокращения побочных реакций на лекарства. Это помогло компании расширить портфель продуктов.
- В феврале 2022 года PacBio, ведущий поставщик высококачественных и высокоточных платформ секвенирования, объявила, что поддерживает Больницу для больных детей (SickKids) в Торонто, Канада, в использовании HiFi секвенирования всего генома (HiFi WGS) для потенциального выявления генетических вариантов, которые могут быть связаны с медицинскими и развивающимися состояниями. Образцы, которые исследуются с помощью HiFi WGS, ранее секвенировались с использованием технологии секвенирования ДНК с коротким считыванием, но до сих пор не идентифицируют вариант, вызывающий заболевание. Это помогло компании расширить использование ее продукции.
- В июле 2022 года, согласно новому общенациональному исследованию почти 2000 ветеранов, проведенному Министерством по делам ветеранов США (VA), показатели ремиссии при большом депрессивном расстройстве (MDD) значительно улучшились, когда врачи получили доступ к результатам психотропных тестов GeneSight от Myriad Genetics, Inc. в крупнейшем в истории рандомизированном контролируемом исследовании психического здоровья PGx. Это помогло компании продемонстрировать свой прогресс в фармакогенетическом тестировании.
- В январе 2021 года myDNA Life Australia Pty Ltd объявила о слиянии с американской компанией по тестированию ДНК для потребителей FamilyTreeDNA из Хьюстона и ее материнской компанией. Gene by Gene за революцию в области фармакогеномики, сделав по-настоящему персонализированную медицину реальностью, прежде чем расшириться в нутригеномику для предоставления действенных персонализированных рекомендаций по питанию, фитнесу и уходу за кожей. Это помогло компании расширить свой бизнес.
Фармакогенетическое тестирование в психиатрии/депрессии на Ближнем Востоке и в Африке. Сфера применения
Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке сегментируется по типу, типу теста, типу гена, типу пациента, продукту, конечному пользователю и каналу сбыта. Рост среди сегментов помогает вам анализировать нишевые карманы роста и стратегии для выхода на рынок и определять ваши основные области применения и разницу в ваших целевых рынках.
- Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке, по типу
- Беспокойство
- Расстройства настроения
- депрессия
- Биполярные расстройства
- Психотические расстройства
- Расстройства пищевого поведения
На основе типа рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке сегментируется на тревожность, расстройства настроения, депрессию, биполярные расстройства, психотические расстройства и расстройства пищевого поведения.
- Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке, по типу теста
- Секвенирование всего генома
- Тесты на основе хромосомного анализа
На основе типа теста рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке сегментируется на полногеномное секвенирование и тесты на основе хромосомного анализа.
- Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке по типу гена
- CYP2C19
- CYP2C9 И VKORC1
- CYP2D6
- HLA-B
- HTR2A/C
- HLA-А
- CYP3A4
- SLC6A4
- МТХФР
- КОМТ
- ДРУГИЕ
На основе типа гена рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке сегментируется на CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT и другие.
- Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке, по типу пациента
- Ребенок
- Взрослый
- гериатрический
В зависимости от типа пациента рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке сегментируется на детский, взрослый и гериатрический.
- Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке, по продуктам
- Инструменты
- Расходные материалы
- Программное обеспечение и услуги
По типу продукции рынок фармакогенетического тестирования в психиатрии/депрессии на Ближнем Востоке и в Африке сегментируется на инструменты, расходные материалы, а также программное обеспечение и услуги.
- Фармакогенетическое тестирование на рынке психиатрии/депрессии на Ближнем Востоке и в Африке, конечным пользователем
- Больницы и клиники
- Лаборатории диагностики
- Академические и научно-исследовательские институты
- Другие
On the basis of end users, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into hospitals and clinics, diagnostics laboratories, academic and research institutes, and others.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Distribution Channel
- Direct Tender
- Third-Party Distribution
- Hospital Pharmacy
- Others

On the basis of distribution channel, the Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into direct tender, third party distribution hospital pharmacy, and others.
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Regional Analysis/Insights
The Middle East & Africa pharmacogenetics testing in psychiatry/depression market is analyzed, and market size information is provided by the type, test type, gene type, patient type, product, end user, and distribution channel. The countries covered in this market report are UAE, Israel, South Africa, Egypt, Kuwait, and rest of the Middle East & Africa.
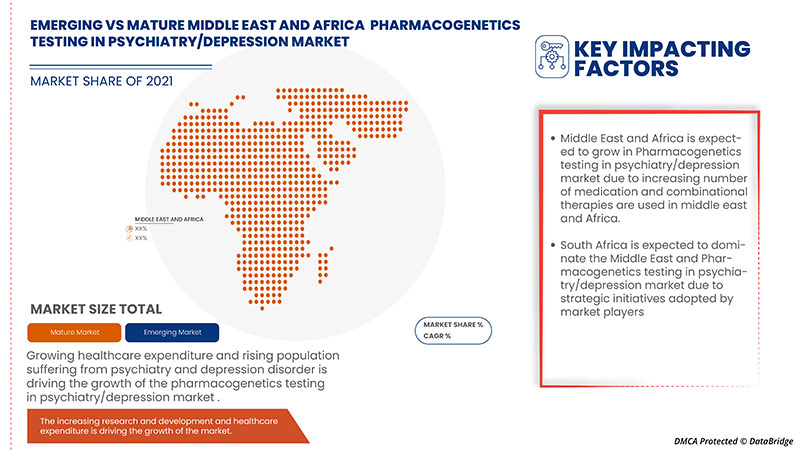
In 2022, Middle East & Africa is dominating due to the presence of key market players in the largest consumer market with high GDP. South Africa is expected to grow due to the rise in technological advancement in pharmacogenetics testing.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Middle East & African brands, the challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Share Analysis
Middle East & Africa pharmacogenetics testing in psychiatry/depression market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Middle East & Africa pharmacogenetics testing in psychiatry/depression market.
Some of the major players operating in the Middle East & Africa pharmacogenetics testing in psychiatry/depression market are
- Genelex (Part of Invitae corporation)
- Genewiz (Part of Azenta Life Sciences)
- MD Labs
- BiogeneiQ, Inc.
- ONEOME, LLC
- Myriad Genetics, Inc.
- GenXys
- Castle Biosciences, Inc.
- PacBio
- QIAGEN
- Thermo Fisher Scientific Inc.
- AB-Biotics.S.A.
- Coriell Life Sciences
- Eurofins Scientific
- Illumina, Inc.
- Dynamic DNA Laboratories
- STADAPHARM GmbH
- Color
- Cnsdose
- Genomind, Inc.
- Healthspek
- myDNA Life Australia Pty Ltd.
- HudsonAlpha
- Sonic Healthcare Limited
Research Methodology: Middle East & Africa pharmacogenetics testing in psychiatry/depression market
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Middle East & Africa vs Regional, and Vendor Share Analysis. Please request an analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Содержание
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PESTEL ANALYSIS
3.2 PORTER'S FIVE FORCES MODEL
3.3 INDUSTRIAL INSIGHTS:
3.4 PIPELINE ANALYSIS FOR MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
3.5 EPIDEMIOLOGY
4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, REGULATIONS
4.1 UNITED STATES (U.S.)
4.1.1 ROLE OF FDA
4.1.2 ROLE OF CDC AND HCFA
4.2 EUROPEAN UNION (EU)
4.3 FRANCE
4.4 AUSTRALIA
4.5 SOUTH KOREA
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 INCREASE IN THE NUMBER OF PATIENTS SUFFERING FROM PSYCHIATRIC AND DEPRESSION DISORDER
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 RISE IN DEMAND FOR PERSONALIZED AND PRECISION MEDICINE
5.2 RESTRAINTS
5.2.1 LACK OF SKILLED MEDICAL AND GENOMIC EXPERT
5.2.2 LACK OF STRONG CLINICAL EVIDENCE
5.2.3 HIGH COST ASSOCIATED WITH THE DIAGNOSIS
5.3 OPPORTUNITIES
5.3.1 RISING ADVANCEMENTS IN TECHNOLOGY
5.3.2 INCREASING NUMBER OF KEY PLAYERS IN MARKET
5.4 CHALLENGES
5.4.1 STRICT GOVERNMENT REGULATION ON NEW PRODUCTS AND INSTRUMENTS APPROVAL
5.4.2 DEARTH OF SKILLED PERSONNEL
6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
6.1 OVERVIEW
6.2 ANXIETY
6.3 DEPRESSION
6.4 MOOD DISORDERS
6.5 BIPOLAR DISORDERS
6.6 EATING DISORDERS
6.7 PSYCHOTIC DISORDERS
7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT
7.1 OVERVIEW
7.2 CONSUMABLES
7.2.1 WHOLE GENOME SEQUENCING KITS
7.2.2 CHROMOSOMAL ARRAY BASED KITS
7.3 INSTRUMENTS
7.4 SOFTWARE AND SERVICES
8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 WHOLE GENOME SEQUENCING
8.2.1 EXOME SEQUENCING
8.2.1.1 SNP
8.2.1.2 CNV
8.2.1.3 RARE PTV MUTATION
8.2.1.4 ULTRA PTV MUTATION
8.2.1.5 OTHERS
8.2.2 KARYOTYPE
8.2.2.1 SNP
8.2.2.2 CNV
8.2.2.3 RARE PTV MUTATION
8.2.2.4 ULTRA PTV MUTATION
8.2.2.5 OTHERS
8.2.3 LOW COVERAGE WGS
8.2.3.1 SNP
8.2.3.2 CNV
8.2.3.3 RARE PTV MUTATION
8.2.3.4 ULTRA PTV MUTATION
8.2.3.5 OTHERS
8.2.4 DEEP COVERAGE WGS
8.2.4.1 SNP
8.2.4.2 CNV
8.2.4.3 RARE PTV MUTATION
8.2.4.4 ULTRA PTV MUTATION
8.2.4.5 OTHERS
8.2.5 MICROARRAY
8.2.5.1 SNP
8.2.5.2 CNV
8.2.5.3 RARE PTV MUTATION
8.2.5.4 ULTRA PTV MUTATION
8.2.5.5 OTHERS
8.2.6 OTHERS
8.3 CHROMOSOMAL ARRAY BASED TESTS
8.3.1 MICRODELETIONS
8.3.2 MICRO DUPLICATIONS
9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE
9.1 OVERVIEW
9.2 CYP2C19
9.3 CYP2C9 AND VKORC1
9.4 CYP2D6
9.5 HLA-B
9.6 HTR2A/C
9.7 HLA-A
9.8 CYP3A4
9.9 SLC6A4
9.1 MTHFR
9.11 COMT
9.12 OTHERS
10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 ADULT
10.3 GERIATRIC
10.4 CHILD
11 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET , BY END USER
11.1 OVERVIEW
11.2 HOSPITAL AND CLINICS
11.2.1 DRUG EFFECTIVENESS
11.2.2 SIDE EFFECTS
11.2.3 DOSAGE
11.3 DIAGNOSTICS LABORATORIES
11.3.1 DRUG EFFECTIVENESS
11.3.2 SIDE EFFECTS
11.3.3 DOSAGE
11.4 ACADEMIC AND RESEARCH INSTITUTES
11.4.1 DRUG EFFECTIVENESS
11.4.2 SIDE EFFECTS
11.4.3 DOSAGE
11.5 OTHERS
12 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 THIRD PARTY DISTRIBUTION
12.4 HOSPITAL PHARMACY
12.5 OTHERS
13 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SOUTH AFRICA
13.1.2 SAUDI ARABIA
13.1.3 U.A.E
13.1.4 ISRAEL
13.1.5 EGYPT
13.1.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 THERMO FISHER SCIENTIFIC INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 RECENT FINANCIALS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 ILLUMINA, INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 MYRIAD GENETICS, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 SONIC HEALTHCARE LIMITED
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 QIAGEN
16.5.1 COMPANY SNAPSHOT
16.5.2 RECENT FINANCIALS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 EUROFINS SCIENTIFIC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AB-BIOTICS, S.A.
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 BIOGENIQ INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 CASTLE BIOSCIENCE, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CNSDOSE
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 COLOR HEALTH, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CORIELL LIFE SCIENCES
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENTS
16.13 DYNAMIC DNA LABORATORIES
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 GENELEX (SUBSIADIARY OF INVITAE CORPORATION.)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 GENEWIZ (PART OF AZENTA LIFE SCIENCES)
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GENOMIND, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 GENXYS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 HEALTHSPEK
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 HUDSONALPHA
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 MD LABS
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 MYDNA LIFE AUSTRALIA PTY LTD.
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 ONEOME, LLC
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENTS
16.23 PACBIO
16.23.1 COMPANY SNAPSHOT
16.23.2 REVENUE ANALYSIS
16.23.3 PRODUCT PORTFOLIO
16.23.4 RECENT DEVELOPMENTS
16.24 STADAPHARM GMBH
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Список таблиц
TABLE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA ANXIETY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA DEPRESSION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA MOOD DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA BIPOLAR DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA EATING DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA PSYCHOTIC DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 10 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 13 MIDDLE EAST & AFRICA INSTRUMENTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA SOFTWARE AND SERVICES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA CYP2C19 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA CYP2C9 AND VKORC1 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA CYP2D6 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA HLA-B IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA HTR2A/C IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HLA-A IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA CYP3A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA SLC6A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA MTHFR IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA COMT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA ADULT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA GERIATRIC IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA CHILD IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA DIRECT TENDER IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA THIRD PARTY DISTRIBUTION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA HOSPITAL PHARMACY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 54 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 55 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 57 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 58 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 59 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 60 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 61 MIDDLE EAST AND AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 62 MIDDLE EAST AND AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 63 MIDDLE EAST AND AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 64 MIDDLE EAST AND AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 65 MIDDLE EAST AND AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 66 MIDDLE EAST AND AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 67 MIDDLE EAST AND AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 69 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 70 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 MIDDLE EAST AND AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 72 MIDDLE EAST AND AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 73 MIDDLE EAST AND AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 77 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 78 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 79 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 81 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 82 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 83 SOUTH AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 84 SOUTH AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 85 SOUTH AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 86 SOUTH AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 87 SOUTH AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 88 SOUTH AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 89 SOUTH AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 91 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 92 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 93 SOUTH AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 SOUTH AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 SOUTH AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 96 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 99 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 100 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 101 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 103 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 104 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 105 SAUDI ARABIA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 106 SAUDI ARABIA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 107 SAUDI ARABIA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 108 SAUDI ARABIA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 109 SAUDI ARABIA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 110 SAUDI ARABIA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 111 SAUDI ARABIA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 113 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 114 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 115 SAUDI ARABIA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 116 SAUDI ARABIA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 117 SAUDI ARABIA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 118 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 119 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 120 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 122 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 123 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 124 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 125 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 126 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 127 UAE WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 128 UAE EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 129 UAE KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 130 UAE LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 131 UAE DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 132 UAE MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 133 UAE CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 135 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 136 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 137 UAE HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 138 UAE DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 139 UAE ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 140 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 141 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 143 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 144 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 145 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 146 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 147 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 148 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 149 ISRAEL WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 150 ISRAEL EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 151 ISRAEL KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 152 ISRAEL LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 153 ISRAEL DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 154 ISRAEL MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 155 ISRAEL CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 157 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 158 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 159 ISRAEL HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 160 ISRAEL DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 161 ISRAEL ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 162 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 163 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 164 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 165 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 166 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 167 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 168 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 169 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 170 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 171 EGYPT WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 172 EGYPT EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 173 EGYPT KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 174 EGYPT LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 175 EGYPT DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 176 EGYPT MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 177 EGYPT CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 179 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 180 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 181 EGYPT HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 182 EGYPT DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 183 EGYPT ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 184 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 185 REST OF MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
Список рисунков
FIGURE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 11 THE INCREASING ADOPTION OF PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION DEVICES AND PROCEDURES AND RISING PREFERENCE FOR NON-SURGICAL PROCEDURES ARE EXPECTED TO DRIVE THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SOFTWARE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN THE PSYCHIATRY/DEPRESSION MARKET
FIGURE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021
FIGURE 16 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 17 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 18 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 19 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2021
FIGURE 20 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 21 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 22 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 23 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2021
FIGURE 24 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 26 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 27 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2021
FIGURE 28 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2022-2029 (USD MILLION)
FIGURE 29 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, CAGR (2022-2029)
FIGURE 30 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, LIFELINE CURVE
FIGURE 31 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2021
FIGURE 32 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 33 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 34 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 35 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2021
FIGURE 36 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, CAGR (2022-2029)
FIGURE 38 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, LIFELINE CURVE
FIGURE 39 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2021)
FIGURE 44 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021)
FIGURE 45 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE (2022-2029)
FIGURE 48 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY SHARE 2021 (%)

Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.













