Middle East And Africa Car T Cell Therapy Treatment Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
16.22 Million
USD
138.11 Million
2024
2032
USD
16.22 Million
USD
138.11 Million
2024
2032
| 2025 –2032 | |
| USD 16.22 Million | |
| USD 138.11 Million | |
|
|
|
|
Сегментация рынка терапии с использованием CAR-T-клеток MEA, по продукту (аутологичные CAR-T-клетки и аллогенные CAR-T-клетки), структура (CAR-T-клетки первого поколения, Car-T-клетки второго поколения, CAR-T-клетки третьего поколения и CAR-T-клетки четвертого поколения), целевые антигены (антигены солидных опухолей, антигены гематологических злокачественных новообразований и другие), бренд (Yescarta, Kymriah, Tecartus и другие), терапевтическое применение (диффузная крупноклеточная В-клеточная лимфома, фолликулярная лимфома, острый лимфобластный лейкоз (ОЛЛ), лимфома из клеток мантийной зоны, множественная миелома, гематологические злокачественные новообразования, рак легких, хронический лимфоцитарный лейкоз, рак желудка, рак поджелудочной железы, рак молочной железы и другие), конечный пользователь (больницы, специализированные Клиники и другие), канал сбыта (больницы, аптеки и другие) - тенденции отрасли и прогноз до 2032 года
Размер рынка лечения с помощью клеточной терапии CAR-T на MEA
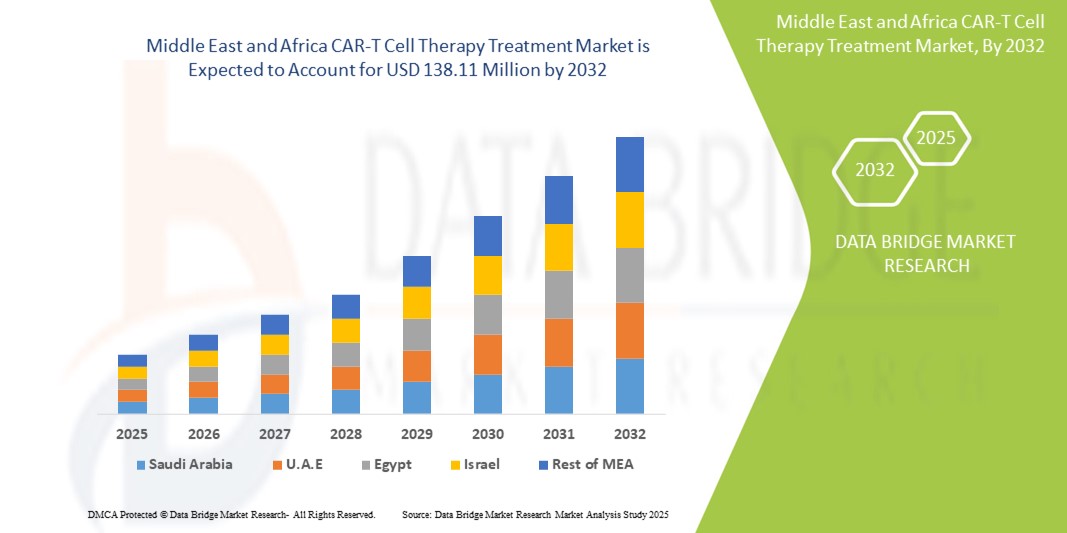
- Объем рынка терапии CAR-T-клетками на Ближнем Востоке и в Африке оценивается в 16,22 млн долларов США в 2024 году и, как ожидается , достигнет 138,11 млн долларов США к 2032 году при среднегодовом темпе роста 30,7% в течение прогнозируемого периода.
- Рост рынка в значительной степени обусловлен растущей распространенностью гематологических злокачественных новообразований и прогрессивным расширением инфраструктуры передовой клеточной и генной терапии в регионе Ближнего Востока и Африки (MEA). Улучшение нормативно-правовой базы, растущие инвестиции в биотехнологии и создание исследовательских и производственных центров CAR-T ускоряют клиническую разработку и доступность клеточной терапии CAR-T
- Кроме того, растущий спрос пациентов на целевые, персонализированные и высокоэффективные варианты лечения позиционирует терапию CAR-T-клетками как прорывной подход в лечении рака. Эти сходящиеся факторы ускоряют внедрение решений MEA CAR-T-клеточной терапии, тем самым значительно ускоряя рост отрасли на ключевых рынках, таких как ОАЭ, Саудовская Аравия, Южная Африка и Египет.
Анализ рынка лечения с помощью клеточной терапии CAR-T на MEA
- Терапия CAR-T-клетками, инновационная иммунотерапия, которая перепрограммирует Т-клетки пациента для распознавания и атаки раковых клеток, все чаще становится критически важным подходом к лечению гематологических злокачественных новообразований в регионе Ближнего Востока и Африки благодаря своей высокой эффективности, персонализированному характеру и способности вызывать стойкие ремиссии в рецидивирующих или рефрактерных случаях.
- Растущий спрос на терапию CAR-T-клетками обусловлен в первую очередь растущей распространенностью рака крови, такого как лейкемия и лимфома, повышением осведомленности среди медицинских работников и пациентов, а также расширением клинических исследований в регионе Ближнего Востока и Африки.
- ОАЭ доминировали на рынке лечения CAR-T-клеточной терапией на Ближнем Востоке и в Африке с самой большой долей выручки в 26,8% в 2024 году, характеризуясь развитой инфраструктурой здравоохранения, ранним внедрением инновационных методов лечения и значительными инвестициями в персонализированную терапию рака.
- Ожидается, что Израиль станет самой быстрорастущей страной на рынке лечения CAR-T-клеточной терапией на Ближнем Востоке и в Африке с прогнозируемым среднегодовым темпом роста 17,5% в период с 2025 по 2032 год, что обусловлено передовыми исследованиями в области иммуноонкологии, расширяющейся биотехнологической экосистемой и высокой активностью клинических испытаний в области клеточной и генной терапии.
- Антигены в сегменте гематологических злокачественных новообразований доминировали на рынке терапии CAR-T-клетками на Ближнем Востоке и в Африке с долей рынка 66,4% в 2024 году, что обусловлено успехом терапии CAR-T при раке крови, таком как лейкемия, лимфома и множественная миелома.
Область применения отчета и сегментация рынка терапии CAR-T-клетками MEA
|
Атрибуты |
MEA CAR-T-клеточная терапия. Ключевые сведения о рынке |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Ближний Восток и Африка
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, анализ цен, анализ доли бренда, опрос потребителей, демографический анализ, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья/расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка лечения с помощью клеточной терапии CAR-T MEA
« Растущий доступ к передовым методам иммунотерапии на Ближнем Востоке и в Африке »
- Значительная и ускоряющаяся тенденция на рынке терапии CAR-T-клеток на Ближнем Востоке и в Африке — это растущий доступ к передовым иммунотерапиям по всему региону, особенно в городских медицинских центрах. Больницы и специализированные клиники на Ближнем Востоке и в некоторых частях Африки все чаще принимают клеточную терапию рака, что отражает сдвиг в сторону точной медицины
- Например, несколько онкологических центров в таких странах, как Объединенные Арабские Эмираты (ОАЭ) и Саудовская Аравия, сотрудничают с международными биотехнологическими фирмами для изучения клинических испытаний и протоколов лечения CAR-T. Это сотрудничество позволяет местным врачам предоставлять передовые методы лечения, ранее ограниченные западными рынками.
- Растущая региональная осведомленность о персонализированном лечении онкологии влияет как на государственных, так и на частных поставщиков медицинских услуг, чтобы включить CAR-T в долгосрочные стратегии лечения рака. Рост пропаганды и кампаний по повышению осведомленности пациентов вокруг гематологических злокачественных новообразований также играет роль в росте рынка
- Кроме того, регион MEA становится свидетелем роста поддерживаемых правительством инициатив, направленных на улучшение показателей выживаемости при раке, таких как национальные программы по контролю рака и инфраструктуры геномного тестирования рака. Эти усилия имеют решающее значение для выявления пациентов, подходящих для терапии CAR-T-клетками, и упрощения доступа к лечению.
- Академические медицинские центры в таких странах, как Южная Африка и Египет, в настоящее время изучают модели партнерства с многонациональными биофармацевтическими компаниями для создания исследовательских и производственных центров CAR-T, что еще больше повысит региональную доступность этой терапии.
- Сочетание роста заболеваемости раком, развития медицинской инфраструктуры и нормативной поддержки создает благоприятную среду для расширения рынка терапии CAR-T на Ближнем Востоке и в Африке.
Динамика рынка лечения с помощью клеточной терапии CAR-T на MEA
Водитель
«Растущая потребность в связи с ростом заболеваемости раком и достижениями в области прецизионной медицины»
- Растущая распространенность гематологических злокачественных новообразований, таких как диффузная крупноклеточная В-клеточная лимфома, множественная миелома и острый лимфобластный лейкоз на Ближнем Востоке и в Африке (MEA), является важным фактором растущего спроса на терапию CAR-T-клетками. Рост заболеваемости раком и неудовлетворенная потребность в эффективных, долгосрочных методах лечения побуждают системы здравоохранения внедрять передовые иммунотерапии
- Например, в марте 2024 года региональные поставщики медицинских услуг в Саудовской Аравии и ОАЭ сотрудничали с глобальными биотехнологическими фирмами для создания центров терапии CAR-T, сосредоточившись на повышении доступности и результатов для пациентов. Такие инициативы отражают стратегическую приверженность региона интеграции передовых клеточных методов лечения в национальные пути лечения рака
- Кроме того, регион MEA наблюдает рост инвестиций в персонализированную медицину, которая поддерживает применение CAR-T-терапии, адаптированной к индивидуальным профилям опухолей пациентов. Доступность аутологичных CAR-T-продуктов, таких как Yescarta и Kymriah, способствует раннему внедрению в специализированных больницах и научно-исследовательских институтах
- Расширение государственных бюджетов здравоохранения и благоприятная нормативно-правовая база, особенно в странах ССЗ, способствуют клиническим испытаниям и коммерческим одобрениям продуктов CAR-T. Растущая осведомленность среди онкологов и пациентов относительно эффективности этих методов лечения в рецидивирующих или рефрактерных случаях еще больше подпитывает расширение рынка
Сдержанность/Вызов
« Высокие затраты на лечение и ограниченная инфраструктура на развивающихся рынках »
- Одной из самых важных проблем, сдерживающих рынок терапии MEA CAR-T Cell, является высокая стоимость терапии, которая может варьироваться от 350 000 до 500 000 долларов США за цикл лечения. Эта стоимость включает сбор клеток, производство, больничный уход и последующее наблюдение, что создает значительную нагрузку на государственные системы здравоохранения и незастрахованных пациентов.
- Например, в настоящее время в нескольких африканских странах отсутствует инфраструктура для производства и хранения CAR-T-клеток, что приводит к зависимости от международной логистики и длительному времени выполнения заказов. Это влияет на своевременное назначение терапии и ставит под угрозу результаты, особенно в случае агрессивных видов рака.
- Кроме того, нехватка обученного клинического персонала и сертифицированных учреждений для проведения CAR-T терапии создает препятствие для широкого доступа. Ограниченные диагностические возможности для выявления подходящих пациентов еще больше задерживают начало лечения
- Чтобы преодолеть эти препятствия, участники рынка сосредотачиваются на разработке децентрализованных производственных подразделений CAR-T и создании программ обучения в сотрудничестве с региональными правительствами. Усилия по снижению производственных затрат с помощью аллогенных (готовых) платформ CAR-T-клеток также могут расширить доступность в странах с низким и средним уровнем дохода в регионе MEA
- Решение проблем возмещения расходов, регуляторных сложностей и логистических ограничений посредством региональных партнерств и инновационных моделей ценообразования будет иметь решающее значение для долгосрочного успеха рынка терапии CAR-T-клетками на Ближнем Востоке и в Африке.
Область применения терапии CAR-T-клетками на рынке MEA
Рынок терапии CAR-T-клетками MEA сегментирован на четыре основных сегмента в зависимости от продукта, структуры, целевых антигенов и терапевтического применения.
• По продукту
На основе продукта рынок терапии CAR-T-клетками MEA сегментирован на аутологичные CAR-T-клетки и аллогенные CAR-T-клетки. Сегмент аутологичных CAR-T-клеток доминировал на рынке с наибольшей долей выручки в 72,3% в 2024 году, что обусловлено снижением иммуногенности и преимуществами персонализации.
Ожидается, что сегмент аллогенных CAR-T-клеток продемонстрирует самые высокие среднегодовые темпы роста в 24,8% в период с 2025 по 2032 год благодаря их доступности и экономической эффективности.
• По структуре
На основе структуры рынок терапии CAR-T-клетками MEA сегментирован на клетки CAR-T первого поколения, клетки CAR-T второго поколения, клетки CAR-T третьего поколения и клетки CAR-T четвертого поколения. Сегмент клеток CAR-T второго поколения занимал наибольшую долю рынка в 58,9% в 2024 году благодаря их повышенной эффективности и широкому клиническому применению.
Ожидается, что сегмент CAR-T-клеток четвертого поколения будет расти самыми быстрыми темпами среднегодового темпа роста в 26,1% в течение прогнозируемого периода, что обусловлено передовыми технологиями редактирования генов и возможностями многоцелевого воздействия.
• По целевым антигенам
На основе целевых антигенов рынок терапии CAR-T-клетками MEA сегментирован на антигены солидных опухолей, антигены гематологических злокачественных новообразований и др. На антигены гематологических злокачественных новообразований пришлась наибольшая доля выручки в 66,4% в 2024 году, что обусловлено успехом терапии CAR-T при раке крови.
Ожидается, что в течение прогнозируемого периода количество антигенов в солидных опухолях будет расти с максимальным среднегодовым темпом роста в 25,7%, что обусловлено продолжающимися клиническими испытаниями и инновациями в области воздействия на микросреду опухоли.
• По бренду
На основе бренда рынок лечения клеточной терапией CAR-T на базе MEA сегментируется на yescarta, kymriah, tecartus и др. Yescarta занимала самую большую долю рынка в 41,2% в 2024 году, с сильным внедрением для DLBCL и других лимфом.
Ожидается, что Tecartus зарегистрирует самый быстрый среднегодовой темп роста в 22,9% в период с 2025 по 2032 год, что будет обусловлено его результатами в лечении мантийноклеточной лимфомы.
• По терапевтическому применению
На основе терапевтического применения рынок лечения с помощью MEA CAR-T-клеточной терапии сегментирован на диффузную В-крупноклеточную лимфому, фолликулярную лимфому, острый лимфобластный лейкоз (ОЛЛ), мантийноклеточную лимфому, множественную миелому, гематологические злокачественные новообразования, рак легких, хронический лимфолейкоз, рак желудка, рак поджелудочной железы, рак молочной железы и др. Диффузная В-крупноклеточная лимфома (ДККЛ) заняла самую большую долю в 36,5% в 2024 году, что обусловлено ранними одобрениями и успешными результатами лечения пациентов.
Ожидается, что в прогнозируемый период заболеваемость множественной миеломой будет расти самыми быстрыми темпами среднегодового темпа роста на уровне 28,3%, что обусловлено выводом на рынок новых препаратов CAR-T, нацеленных на BCMA, на Ближнем Востоке и в Африке.
• Конечным пользователем
На основе конечного пользователя рынок лечения клеточной терапией CAR-T на Ближнем Востоке и в Африке сегментируется на больницы, специализированные клиники и т. д. Больницы лидировали в сегменте с долей рынка 69,8% в 2024 году благодаря своей способности поддерживать протоколы инфузии CAR-T, мониторинга и восстановления.
Ожидается, что в течение прогнозируемого периода число специализированных клиник будет расти с наивысшим среднегодовым темпом роста в 21,6%, что будет обусловлено децентрализацией и расширением сетей иммунотерапии.
• По каналу сбыта
На основе канала дистрибуции рынок лечения клеточной терапией CAR-T на Ближнем Востоке и в Африке сегментируется на больничную аптеку и другие. Сегмент больничной аптеки занимал наибольшую долю рынка в 78,1% в 2024 году, что обусловлено сложной холодовой цепью и требованиями соответствия.
Прогнозируется, что сегмент «Другие» будет расширяться среднегодовыми темпами в 19,5% в течение прогнозируемого периода, поскольку все больше производителей CAR-T оптимизируют дистрибуцию через специализированных поставщиков логистических услуг.
Региональный анализ рынка лечения с помощью CAR-T-клеточной терапии на Ближнем Востоке и в Африке
- ОАЭ доминировали на рынке лечения CAR-T-клеточной терапией на Ближнем Востоке и в Африке с самой большой долей выручки в 26,8% в 2024 году, характеризуясь развитой инфраструктурой здравоохранения, ранним внедрением инновационных методов лечения и значительными инвестициями в персонализированную терапию рака.
- Пациенты и поставщики медицинских услуг в регионе все больше ценят целенаправленную эффективность, персонализированный подход и долгосрочный потенциал ремиссии, предлагаемые терапией CAR-T-клетками при лечении гематологических онкологических заболеваний, таких как лейкемия и лимфома.
- Рост популярности также обусловлен расширением клинических испытаний, улучшением доступа к передовой онкологической помощи и ростом государственных инвестиций в клеточную терапию, что позиционирует терапию CAR-T как революционный вариант на отдельных рынках Ближнего Востока и Африки.
Обзор рынка лечения CAR-T-клеточной терапией в Саудовской Аравии на Ближнем Востоке и в Африке
Рынок терапии CAR-T-клетками в Саудовской Аравии составил 21,3% от доли выручки рынка MEA в 2024 году и, как ожидается, будет расти со значительным среднегодовым темпом роста в течение прогнозируемого периода. Этот рост обусловлен правительственной стратегией трансформации здравоохранения Vision 2030, расширением специализированных онкологических центров и партнерством с международными биотехнологическими фирмами. Рост случаев гематологического рака и общенациональный толчок к принятию точной медицины подпитывают расширение рынка.
Обзор рынка лечения CAR-T-клеточной терапией в ОАЭ на Ближнем Востоке и в Африке
Рынок терапии CAR-T-клетками в ОАЭ занял самую высокую долю рынка в 26,8% в регионе Ближнего Востока и Африки в 2024 году, что обусловлено его значительными инвестициями в передовые методы лечения, высокими расходами на здравоохранение и проактивными разрешениями регулирующих органов. Терапия CAR-T быстро внедряется в ведущих онкологических больницах страны при поддержке сотрудничества с мировыми новаторами, такими как Gilead и Novartis.
Обзор рынка лечения CAR-T-клеточной терапией в Израиле на Ближнем Востоке и в Африке
Израильский рынок лечения клеточной терапией CAR-T с прогнозируемым среднегодовым темпом роста 17,5% с 2025 по 2032 год благодаря своему передовому сектору биотехнологий, сильным академическим возможностям НИОКР и высокому уровню активности клинических испытаний CAR-T. Рынок готов к дальнейшему расширению с ростом внутренних разработок и применения иммунотерапии следующего поколения.
Доля рынка лечения с помощью клеточной терапии CAR-T на MEA
Индустрия лечения с использованием CAR-T-клеток MEA в основном представлена хорошо зарекомендовавшими себя компаниями, среди которых:
- Новартис АГ (Швейцария)
- Gilead Sciences, Inc. (США)
- Компания Bristol-Myers Squibb (США)
- Johnson & Johnson Services, Inc. (США)
- Autolus Therapeutics plc (Великобритания)
- Poseida Therapeutics, Inc. (США)
- Sorrento Therapeutics, Inc. (США)
- Miltenyi Biotec (Германия)
- CARsgen Therapeutics (Китай)
- JW Therapeutics (Shanghai) Co., Ltd. (Китай)
- Корпорация Legend Biotech (Китай)
- Tessa Therapeutics (Сингапур)
- Adaptimmune Therapeutics plc (Великобритания)
- Bluebird Bio, Inc. (США)
- Celyad Oncology SA (Бельгия)
- Аллогенная терапия (США)
- Immatics NV (Германия)
- Pfizer Inc. (США)
Последние разработки на рынке лечения CAR-T-клеточной терапией на Ближнем Востоке и в Африке
- В декабре 2023 года компания Gilead Sciences, Inc. объявила, что FDA США одобрило обновление инструкции по применению препарата Yescarta (аксикабтаген цилолеуцел), включив в нее первичный анализ общей выживаемости (OS) из знаменательного исследования фазы 3 ZUMA-7, показывающего статистически значимое улучшение OS при применении препарата Yescarta по сравнению со стандартным лечением (SOC) в качестве терапии второй линии с излечивающим намерением для пациентов с рецидивирующей или рефрактерной крупноклеточной В-клеточной лимфомой (R/R LBCL) в течение 12 месяцев после завершения терапии первой линии.
- В мае 2022 года компания Bristol-Myers Squibb объявила об одобрении Opdivo plus Yervoy в качестве терапии первой линии для взрослых пациентов Министерством здравоохранения, труда и благосостояния Японии. Это может помочь компании укрепить свой продуктовый портфель
- В феврале 2022 года FDA одобрило препарат CARVYKTI (ciltacabtagene autoleucel) от Janssen для лечения взрослых с рецидивирующей или рефрактерной множественной миеломой после четырех или более предыдущих линий терапии, что стало первой клеточной терапией Janssen. Это подчеркнет приверженность компании совершенствованию своих вариантов лечения онкологии
- В декабре 2021 года Novartis AG подписала соглашение с BeiGene, Ltd. на ociperlimab (BGB-A1217), что расширит возможности компании в области иммуноонкологических исследований и разработок. Это сотрудничество вносит вклад в более широкую инициативу Novartis Oncology по продвижению инноваций в лечении рака путем включения потенциально преобразующей терапии в ее расширяющуюся платформу иммунотерапии.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.