Global Pharmacogenetic Testing Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
610.34 Million
USD
1,346.87 Million
2024
2032
USD
610.34 Million
USD
1,346.87 Million
2024
2032
| 2025 –2032 | |
| USD 610.34 Million | |
| USD 1,346.87 Million | |
|
|
|
|
Глобальный рынок фармакогенетического тестирования по типу (секвенирование всего генома, секвенирование всего экзома, тесты на основе матриц и тесты отдельных генов), тип гена (CYP2C19, CYP2D6, CYP2C9 и VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX и MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT и другие), тип препарата (рецептурные препараты, нутрицевтики, рекреационные наркотики, растительные добавки, витамины и безрецептурные препараты), образец (кровь и слюна), терапевтическая область (кардиология, гастроэнтерология, анестезиология, геномика , эндокринология, иммунология и Гиперчувствительность, дерматология, гинекология, онкология, неврология и другие), применение (клиническая практика, разработка лекарственных средств и регулирование лекарственных средств), конечный пользователь (поставщики медицинских услуг, фармацевтические и биотехнологические компании, исследовательские центры и академические институты и другие), канал сбыта (больничная аптека, розничные аптеки, аптеки почтовой доставки и услуги прямого обслуживания клиентов) — тенденции отрасли и прогноз до 2030 года.
Анализ и размер рынка фармакогенетического тестирования
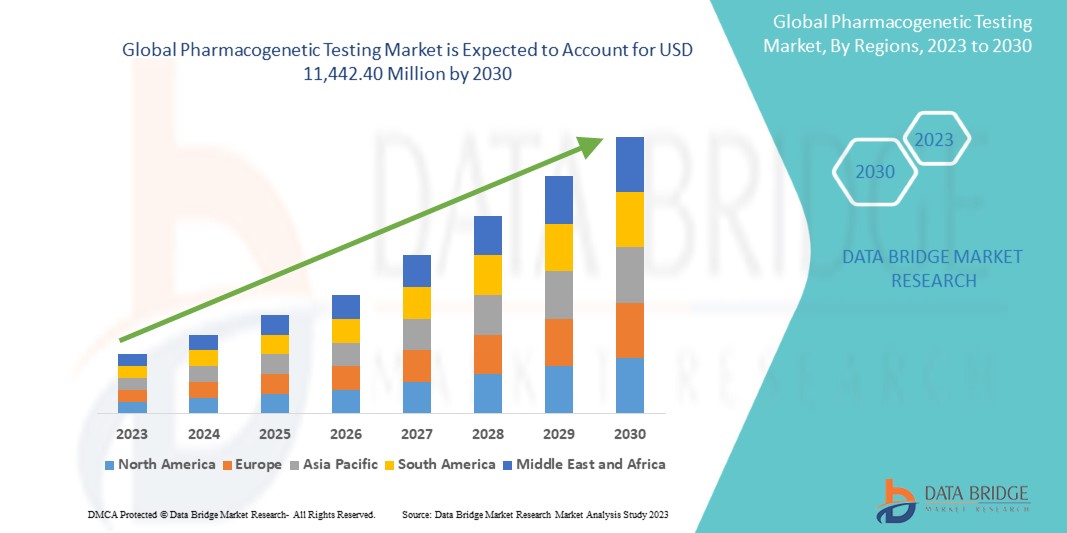
Ожидается, что мировой рынок фармакогенетического тестирования будет расти в прогнозируемый период с 2023 по 2030 год. Развитие рынка обусловлено растущим принятием персонализированной медицины, а это рынок, которому необходим рынок фармакогенетического тестирования.
Интеграция электронных медицинских карт (EHR) является значительным фактором роста рынка. Ожидается, что рост хронических и сложных заболеваний создаст возможности для роста рынка. Data Bridge Market Research анализирует, что рынок растет со среднегодовым темпом роста 8,7% в прогнозируемый период с 2023 по 2030 год и, как ожидается, достигнет 11 442,40 млн долларов США к 2030 году. Ожидается, что прогресс в области геномных технологий будет способствовать расширению рынка.
|
Отчет Метрика |
Подробности |
|
Прогнозируемый период |
2023-2030 |
|
Базовый год |
2022 |
|
Исторический год |
2021 (настраиваемый 2015-2020) |
|
Количественные единицы |
Доход в млн. долл. США |
|
Охваченные сегменты |
Тип (секвенирование всего генома, секвенирование всего экзома , тесты на основе матриц и тесты отдельных генов), тип гена (CYP2C19, CYP2D6, CYP2C9 и VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX и MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT и другие), тип препарата (рецептурные препараты, нутрицевтики, рекреационные наркотики, растительные добавки, витамины и безрецептурные препараты), образец (кровь и слюна), терапевтическая область (кардиология, гастроэнтерология, анестезиология, геномика, эндокринология, иммунология и гиперчувствительность, дерматология, Гинекология, онкология, неврология и другие), применение (клиническая практика, разработка лекарственных препаратов и регулирование лекарственных средств), конечный пользователь (поставщики медицинских услуг, фармацевтические и биотехнологические компании, исследовательские центры и академические институты и другие), канал распространения (больничная аптека, розничные аптеки, аптеки почтовой доставки и услуги прямого обслуживания клиентов) |
|
Страны, охваченные |
США, Канада, Мексика, Франция, Германия, Испания, Италия, Великобритания, Испания, Нидерланды, Россия, Турция, Швейцария, Остальная Европа, Китай, Япония, Таиланд, Индия, Южная Корея, Австралия, Сингапур, Индонезия, Филиппины, Малайзия, Остальная часть Азиатско-Тихоокеанского региона, Бразилия, Аргентина, Остальная часть Южной Америки, ОАЭ, Саудовская Аравия, Южная Африка, Израиль, Египет и Остальная часть Ближнего Востока и Африки |
|
Охваченные участники рынка |
PerkinElmer Inc., Illumina, Inc., Thermo Fisher Scientific Inc., Dynamic DNA Laboratories, Sonic Healthcare, QIAGEN, Eurofins Scientific, 23andMe, Inc, OneOme, BGI, PacBio, MD Labs, GENEWIZ, PGXT, Luminex Corporation и Myriad Genetics, Inc., среди прочих |
Определение рынка фармакогенетического тестирования
Фармакогенетическое тестирование включает в себя услуги, которые изучают генетический профиль человека, чтобы выяснить, как гены человека влияют на реакцию его на определенные препараты. Это специализированное генетическое тестирование выявляет определенные генетические вариации, которые могут влиять на метаболизм, эффективность и потенциальные побочные эффекты различных лекарств. Медицинские специалисты могут настраивать выбор и дозировку лекарств для пациента, чтобы улучшить результаты лечения, уменьшить побочные эффекты и повысить общую эффективность лекарств. Это делается путем знания генетической предрасположенности этого пациента. Качество здравоохранения и результаты для пациента могут улучшиться благодаря этому персонализированному выбору лекарств и подходу к дозировке.
Динамика рынка фармакогенетического тестирования
В этом разделе рассматривается понимание движущих сил рынка, преимуществ, возможностей, ограничений и проблем. Все это подробно обсуждается ниже:
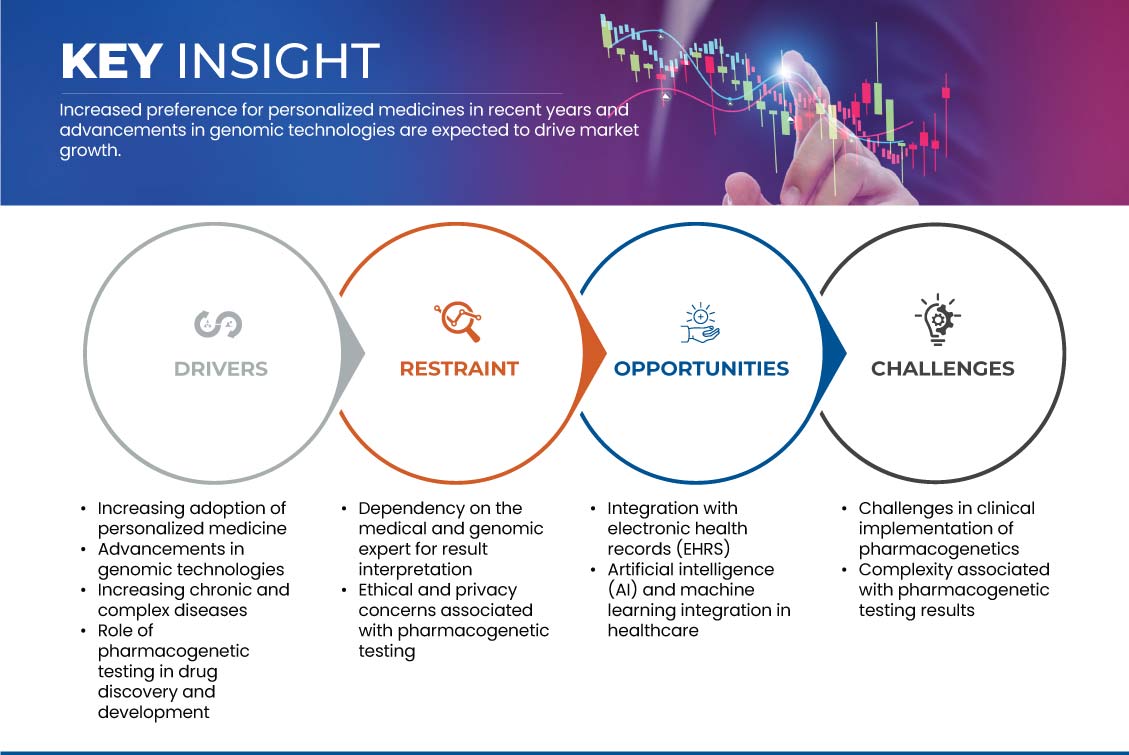
Драйверы
- Растущее внедрение персонализированной медицины
Фармакогенетическое тестирование играет важную роль в персонализированной медицине, подбирая лекарственные препараты под генетический состав человека. Этот инновационный подход включает анализ генетических вариаций человека, которые могут влиять на то, как он метаболизирует и реагирует на определенные лекарства. Эти генетические различия могут влиять на эффективность лекарств и потенциальные побочные эффекты, что делает фармакогенетическое тестирование ценным инструментом для персонализированных планов лечения.
Поставщики медицинских услуг могут идентифицировать генетические маркеры, которые определяют, как их организм обрабатывает и использует лекарства, изучая уникальный генетический профиль человека. Эта информация позволяет настраивать выбор лекарств и дозировки для оптимизации результатов лечения. Например, у некоторых людей могут быть генетические варианты, которые делают их более чувствительными к определенным лекарствам, что требует более низких доз для достижения желаемого терапевтического эффекта без побочных реакций. С другой стороны, некоторые генетические вариации могут требовать более высоких доз для того же эффекта. Этот индивидуальный подход сводит к минимуму «пробы и ошибки», часто связанные с выбором лекарств, снижая риск побочных реакций и повышая эффективность лечения. Он позволяет медицинским работникам принимать обоснованные решения и назначать лекарства, которые с большей вероятностью будут хорошо работать для пациентов на основе их генетической предрасположенности. В конечном счете, фармакогенетическое тестирование вносит значительный вклад в парадигму персонализированной медицины, революционизируя здравоохранение, продвигая более точные и индивидуализированные стратегии лечения для лучших результатов для пациентов.
Таким образом, рынок выигрывает от этого всплеска спроса, наблюдая постоянный рост НИОКР-активностей для создания более комплексных и точных решений для тестирования. Технологические достижения, такие как секвенирование следующего поколения (NGS) и другие методы молекулярной диагностики, еще больше повышают точность и доступность этих тестов. Результатом является взаимоусиливающая связь между ростом персонализированной медицины и процветающим рынком, причем оба существенно влияют на ландшафт здравоохранения, обещая будущее, в котором лечение будет индивидуальным, эффективным и ориентированным на пациента, что, как ожидается, будет стимулировать рост рынка.
- Достижения в области геномных технологий
Продолжающийся всплеск геномных инноваций значительно повысил точность, скорость и экономическую эффективность генетического тестирования. Секвенирование следующего поколения (NGS) и другие передовые методы молекулярной диагностики стали ключевыми инструментами, революционизирующими то, как анализируется и интерпретируется генетическая информация. Интеграция этих передовых геномных технологий повысила точность и объем фармакогенетического тестирования, что позволило провести комплексную оценку генетического профиля человека. Это, в свою очередь, позволяет медицинским работникам принимать обоснованные решения относительно выбора лекарств, дозировки и стратегий лечения, адаптированных к уникальной генетической структуре пациента.
Более того, технология редактирования генов CRISPR-Cas9 показала многообещающие результаты в изучении функционального воздействия определенных генетических вариантов. Эта технология позволяет исследователям точно модифицировать гены, имитируя определенные генетические вариации и выявляя их влияние на метаболизм и реакцию на лекарства. Эти достижения в совокупности способствуют совершенствованию и расширению рынка, приближая будущее, в котором лекарства будут подбираться индивидуально для каждого человека на основе его уникального генетического кода, что, как ожидается, будет способствовать росту рынка.
Возможность
- Интеграция с электронными медицинскими картами (ЭМК)
Бесшовная интеграция обеспечивает эффективное и централизованное хранение генетических данных наряду с подробными медицинскими картами пациентов. Такое объединение повышает доступность и удобство использования фармакогенетической информации для медицинских работников, позволяя им принимать решения на основе данных в режиме реального времени. Врачи могут легко получать доступ к генетическим данным во время планирования лечения, гарантируя назначение лекарств, соответствующих уникальному генетическому составу пациента. Повышение эффективности, достигаемое благодаря этой интеграции, ускоряет внедрение фармакогенетического тестирования, способствуя улучшению ухода за пациентами, оптимизации рабочих процессов и, в конечном итоге, стимулированию роста на рынке.
Более того, интеграция с EHR облегчает исследования и анализ данных в более широком масштабе. Агрегированные и деидентифицированные генетические данные в EHR могут быть использованы для исследований и клинических исследований, способствуя прогрессу в фармакогеномике. Фармацевтические компании, исследовательские институты и поставщики медицинских услуг могут использовать эти данные для улучшения разработки лекарств, проверки генетических ассоциаций и оптимизации стратегий лечения. Это сотрудничество между рынком и EHR подпитывает инновации. Оно способствует созданию синергетической экосистемы, которая приносит пользу сектору здравоохранения и продвигает рынок вперед за счет расширенных исследований, разработки продуктов и принятия решений на основе данных, что, как ожидается, создаст возможности для роста рынка.
Сдержанность/Вызов
- Зависимость от медицинского и геномного эксперта при интерпретации результатов
Рынок сталкивается с заметным ограничением в виде сильной зависимости от медицинских и геномных экспертов для интерпретации результатов. Сложная природа генетических данных требует специальных знаний и опыта для точного и осмысленного понимания. Правильная интерпретация результатов фармакогенетического тестирования в значительной степени зависит от высококвалифицированных специалистов, включая генетиков, фармакологов и экспертов по геномике. Эта зависимость создает проблему с точки зрения масштабируемости и широкого внедрения фармакогенетического тестирования. Ограниченный пул квалифицированных экспертов, а также время и усилия, необходимые для точной интерпретации, часто задерживают предоставление действенных идей практикующим врачам и пациентам. Более того, эта зависимость от экспертов может привести к увеличению затрат и использованию ресурсов, что препятствует бесшовной интеграции фармакогенетического тестирования в повседневную клиническую практику. Преодоление этого ограничения требует усовершенствования автоматизированных инструментов анализа данных, удобных интерфейсов и комплексных образовательных программ, чтобы расширить возможности более широкого спектра медицинских работников в точной интерпретации и использовании результатов фармакогенетических тестов. Таким образом, рынок открывает огромные перспективы в адаптации лекарственной терапии для людей на основе их генетического состава. Критическая проблема заключается в зависимости от медицинских и геномных экспертов для интерпретации результатов тестов. Сложность генетических данных требует особой экспертизы, что затрудняет интерпретацию результатов и принятие клинических решений, что, как ожидается, будет сдерживать рост рынка.
Недавнее развитие
- В мае 2023 года PerkinElmer Inc. объявила о запуске Revvity, Inc. как поставщика научно обоснованных решений, использующих достижения в области диагностики и естественных наук для улучшения жизни людей по всему миру. Ранее бизнес был связан с PerkinElmer, Inc. Клиенты Revvity представляют различные отрасли, включая фармацевтику и биотехнологии, диагностические лаборатории, академические круги и правительственные организации. Revvity предлагает реагенты, расходные материалы, анализы, приборы и программное обеспечение
- В октябре 2022 года PacBio и Twist Bioscience Corporation объявили о доступности начального портфолио готовых панелей генов с длинными считываниями. Эти фиксированные панели Twist Alliance созданы для эффективного и экономичного захвата целевых областей. Кроме того, клиенты смогут создать панель своей конструкции, которая будет полностью адаптируемой и масштабируемой для секвенирования с использованием считываний PacBio HiFi
Масштаб мирового рынка фармакогенетического тестирования
Глобальный рынок фармакогенетического тестирования сегментирован на восемь заметных сегментов на основе типа, типа гена, типа препарата, образца, терапевтической области, применения, конечного пользователя и канала распространения. Рост среди этих сегментов поможет вам проанализировать основные сегменты роста в отраслях и предоставить пользователям ценный обзор рынка и рыночные идеи для принятия стратегических решений по определению основных рыночных приложений
Тип
- Секвенирование всего генома
- Секвенирование всего экзома
- Тесты на основе массивов
- Тесты отдельных генов
По типу рынок сегментируется на секвенирование всего генома, секвенирование всего экзома, тесты на основе массивов и тесты отдельных генов.
Тип гена
- CYP2C19
- CYP2D6
- CYP2C9 и VKORC1
- CYP1A2
- HLA-B*1502
- HLA-B*5701
- CYP2D
- ОПРМ1
- ONCOTYPE DX® и MAMMAPRINT®
- ДРД3
- Д4Д4
- SLC6A4
- HTR2A/C
- ТМПТ
- Другие
На основе типа гена рынок сегментирован на CYP2C19, CYP2D6, CYP2C9 и VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX и MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT и другие.
Тип препарата
- Лекарства, отпускаемые по рецепту
- Нутрицевтики
- Рекреационные наркотики
- Травяные добавки
- Витамины
- Лекарства, отпускаемые без рецепта
По типу лекарственных средств рынок сегментируется на рецептурные препараты, нутрицевтики, рекреационные наркотики, растительные добавки, витамины и безрецептурные препараты.
Образец
- Кровь
- Слюна
На основе выборки рынок сегментируется на кровь и слюну.
Терапевтическая зона
- Кардиология
- Гастроэнтерология
- Анестезиология
- Геномика
- Эндокринология
- Иммунология и гиперчувствительность
- Дерматология
- Гинекология
- Онкология
- Неврология
- Другие
По терапевтическому направлению рынок сегментирован на кардиологию, гастроэнтерологию, анестезиологию, геномику, эндокринологию, иммунологию и гиперчувствительность, дерматологию, гинекологию, онкологию, неврологию и другие.
Приложение
- Клиническая практика
- Разработка лекарств
- Регулирование оборота наркотиков
По сфере применения рынок сегментируется на клиническую практику, разработку лекарственных средств и регулирование лекарственных средств.
Конечный пользователь
- Поставщики медицинских услуг
- Фармацевтические и биотехнологические компании
- Центры и академические институты
- Другие
По признаку конечного пользователя рынок сегментируется на поставщиков медицинских услуг, фармацевтические и биотехнологические компании, научно-исследовательские центры и академические институты и т. д.
Канал распространения
- Больничная аптека
- Розничные аптеки
- Аптеки с доставкой по почте
- Услуги, оказываемые напрямую клиентам
По каналам сбыта рынок сегментируется на больничные аптеки, розничные аптеки, аптеки, работающие по почте, и службы прямых продаж клиентам.
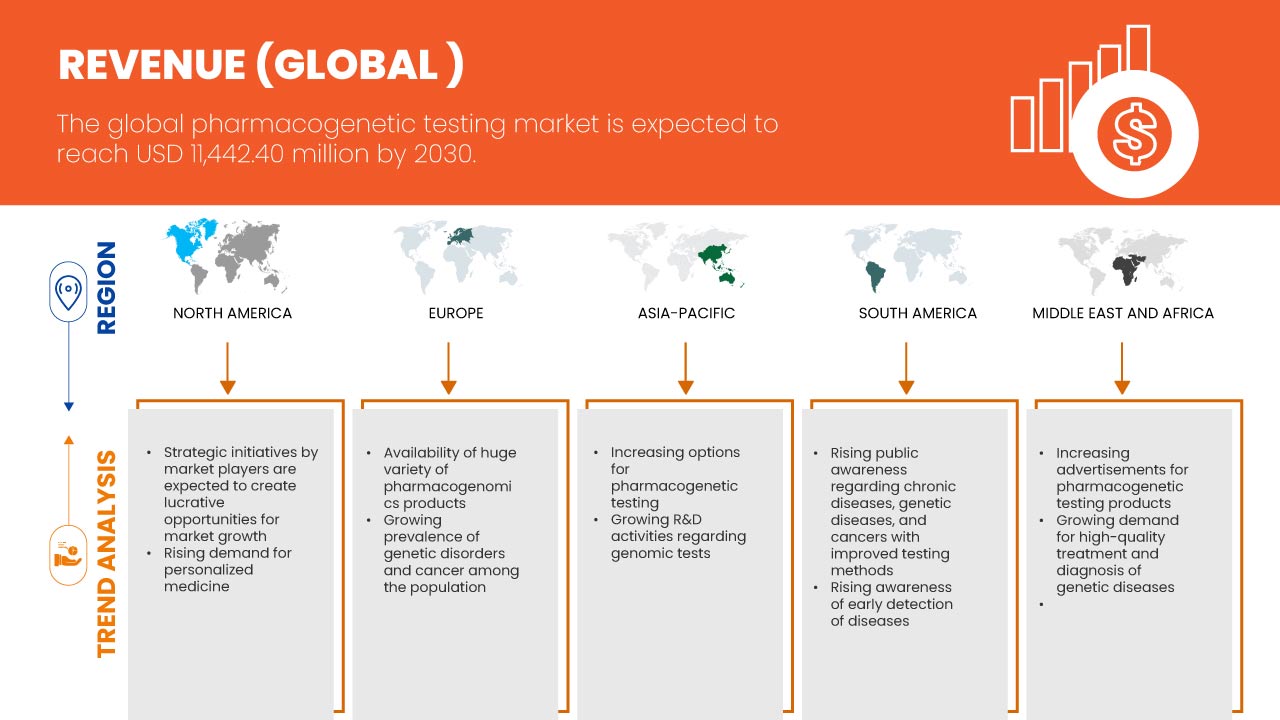
Региональный анализ/информация: рынок фармакогенетического тестирования
Проведен анализ мирового рынка фармакогенетического тестирования, а также предоставлены сведения о размерах рынка и тенденциях на основе типа, типа гена, типа препарата, образца, терапевтической области, применения, конечного пользователя и канала сбыта, как указано выше.
В отчете о рынке рассматриваются следующие страны: США, Канада, Мексика, Франция, Германия, Испания, Италия, Великобритания, Испания, Нидерланды, Россия, Турция, Швейцария, остальные страны Европы, Китай, Япония, Таиланд, Индия, Южная Корея, Австралия, Сингапур, Индонезия, Филиппины, Малайзия, остальные страны Азиатско-Тихоокеанского региона, Бразилия, Аргентина, остальные страны Южной Америки, ОАЭ, Саудовская Аравия, Южная Африка, Израиль, Египет и остальные страны Ближнего Востока и Африки.
Ожидается, что США будут доминировать в регионе Северной Америки из-за своей развитой инфраструктуры и обширной сети высококачественных центров инфраструктуры здравоохранения. Ожидается, что Германия будет доминировать в регионе Европы из-за ее растущих НИОКР, связанных с биотехнологиями и здравоохранением. Ожидается, что Япония будет доминировать в Азиатско-Тихоокеанском регионе из-за ее растущего спроса и растущей осведомленности о рынке фармакогенетического тестирования.
Раздел отчета по странам также содержит отдельные факторы, влияющие на рынок, и изменения в регулировании рынка, которые влияют на текущие и будущие тенденции рынка. Такие данные, как анализ цепочки создания стоимости вверх и вниз по течению, технические тенденции и анализ пяти сил Портера, тематические исследования — вот некоторые из указателей, используемых для прогнозирования рыночного сценария для отдельных стран. Кроме того, при предоставлении прогнозного анализа данных по странам учитываются наличие и доступность региональных брендов и их проблемы из-за большой или малой конкуренции со стороны местных и отечественных брендов, влияние внутренних тарифов и торговых путей.
Анализ конкурентной среды и доли рынка фармакогенетического тестирования
Конкурентная среда мирового рынка фармакогенетического тестирования содержит сведения по конкурентам. Включены сведения о компании, финансах компании, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, региональном присутствии, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта, доминировании приложений. Приведенные выше данные касаются только фокуса компаний на рынке.
К числу основных игроков, работающих на мировом рынке фармакогенетического тестирования, относятся PerkinElmer Inc., Illumina, Inc., Thermo Fisher Scientific Inc., Dynamic DNA Laboratories, Sonic Healthcare, QIAGEN, Eurofins Scientific, 23andMe, Inc, OneOme, BGI, PacBio, MD Labs, GENEWIZ, PGXT, Luminex Corporation и Myriad Genetics, Inc., а также другие.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.