Глобальный рынок диагностики рака яичников по типу продукции (инструменты, наборы и реагенты), типу процедуры (биопсия, медицинская визуализация, тестирование маркеров крови и генетическое тестирование ), типу рака (герминогенная, эпителиальная и стромальноклеточная опухоль), конечному пользователю (центры диагностики рака, больничные лаборатории, научно-исследовательские институты и другие) — тенденции отрасли и прогноз до 2030 года.
Анализ рынка диагностики рака яичников и его понимание
Рак яичников — это тип рака, который образуется в тканях яичника (одной из пары женских репродуктивных желез, в которых образуются яйцеклетки). Большинство видов рака яичников представляют собой либо рак эпителия яичников (рак, который начинается в клетках на поверхности яичника), либо злокачественные опухоли зародышевых клеток (рак, который начинается в яйцеклетках). Тесты и процедуры, используемые для диагностики рака яичников, включают тазовый осмотр, визуализирующие тесты, анализы крови, хирургическое вмешательство и другие. Во время тазового осмотра врач вводит пальцы в перчатках во влагалище и одновременно нажимает рукой на живот, чтобы ощутить (пропальпировать) органы малого таза.
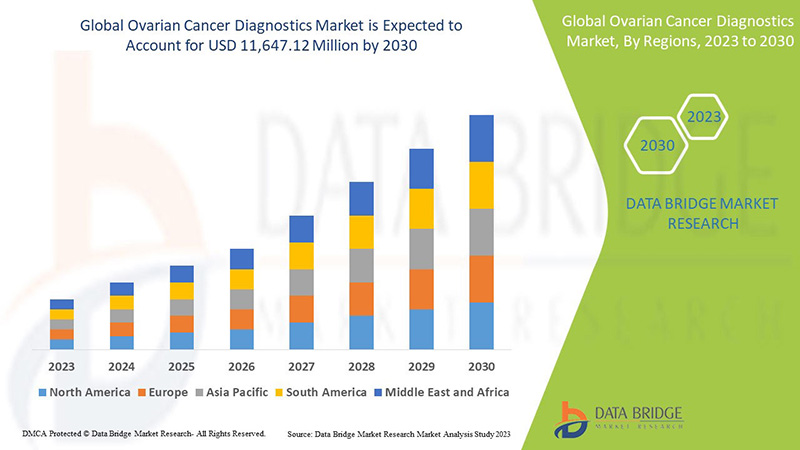
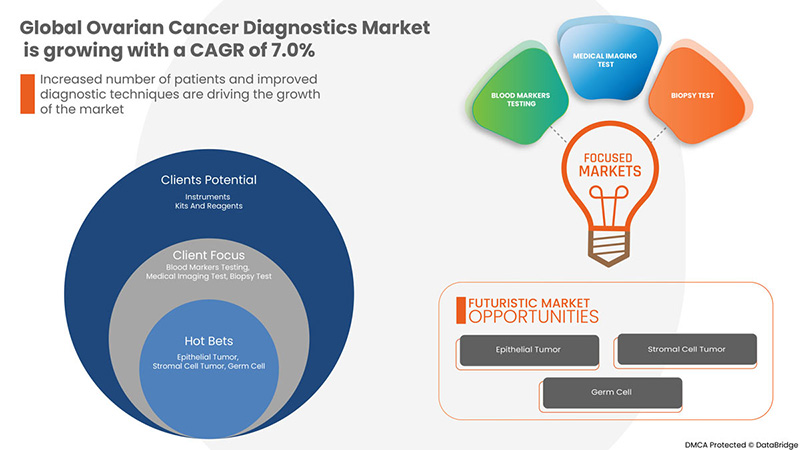
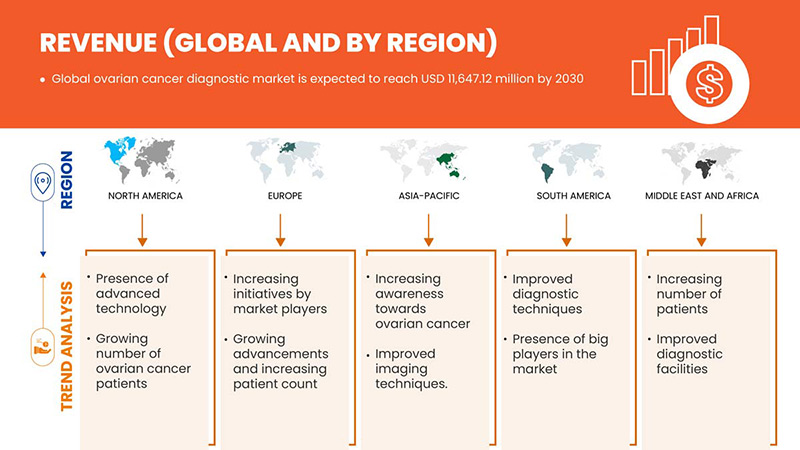
Ожидается, что мировой рынок диагностики рака яичников вырастет в прогнозируемый период с 2023 по 2030 год. По данным Data Bridge Market Research, среднегодовой темп роста рынка составит 7,0% в прогнозируемый период с 2023 по 2030 год, и ожидается, что к 2030 году его объем достигнет 11 647,12 млн долларов США.
|
Отчет Метрика |
Подробности |
|
Прогнозируемый период |
2023-2030 |
|
Базовый год |
2022 |
|
Исторические годы |
2021 (Возможно изменение на 2020-2016) |
|
Количественные единицы |
Доход в млн. долл. США |
|
Охваченные сегменты |
По типу продукта (инструменты, наборы и реагенты), типу процедуры (биопсия, медицинская визуализация, тестирование маркеров крови и генетическое тестирование), типу рака (герминогенная, эпителиальная и стромальноклеточная опухоль), конечному пользователю (центры диагностики рака, больничные лаборатории, научно-исследовательские институты и другие) |
|
Страны, охваченные |
США, Канада и Мексика, Германия, Франция, Великобритания, Италия, Испания, Нидерланды, Россия, Швейцария, Турция, Бельгия и остальные страны Европы, Китай, Япония, Индия, Южная Корея, Австралия, Сингапур, Таиланд, Малайзия, Индонезия, Филиппины и остальные страны Азиатско-Тихоокеанского региона, Бразилия, Аргентина и остальные страны Южной Америки, Южная Африка, Саудовская Аравия, ОАЭ, Египет, Израиль и остальные страны Ближнего Востока и Африки |
|
Охваченные участники рынка |
F. Hoffmann-La Roche Ltd, Tosoh India Pvt. Ltd., Luminex Corporation, Quest Diagnostics Incorporated, Thermo Fisher Scientific Inc., Ngenebio, Abbott, Siemens healthcare private limited, Myriad genetics Inc., Bio-rad laboratorys, Inc., R&d systems, Inc., Foundation medicine, Inc., Biosupply ltd, Lcm genect srl, Inex innovate private limited, Abcam plc., Monobind Inc., Fujirebio, Mp biomedicals, Biovision Inc., Boster biology technology, Biogenix Inc. Pvt. Ltd., Genway biotech и Lifespan biosciences, Inc. |
Определение мирового рынка диагностики рака яичников
Рак яичников чаще всего встречается у женщин в возрасте от 50 до 79 лет. Он становится все более распространенным по мере роста численности гериатрического населения в мире и все большего внимания уделяется раннему выявлению и лечению, что, как ожидается, ускорит развитие рынка диагностики рака яичников. Увеличение государственных инвестиций в повышение осведомленности о раннем выявлении рака, а также увеличение расходов на здравоохранение также будут способствовать росту бизнеса. Ожирение, по-видимому, играет значительную роль в развитии рака яичников. Другие варианты образа жизни, которые могут повысить риск, включают курение, употребление алкоголя и отсутствие детей. Поскольку рак яичников нелегко обнаружить, женщины, которые подвержены риску развития этого заболевания, должны проходить плановое тестирование для раннего выявления заболевания, что позволяет рынку расширяться.
Глобальный рынок диагностики рака яичников растет в прогнозируемом году за счет увеличения числа участников рынка и доступности передовых услуг. Наряду с этим производители занимаются НИОКР для запуска новых услуг на рынке. Растущие исследования в области диагностики и разработки рака яичников еще больше стимулируют рост рынка. Однако трудности в методах скрининга рака яичников могут помешать росту глобального рынка диагностики рака яичников в прогнозируемый период.
Динамика мирового рынка диагностики рака яичников
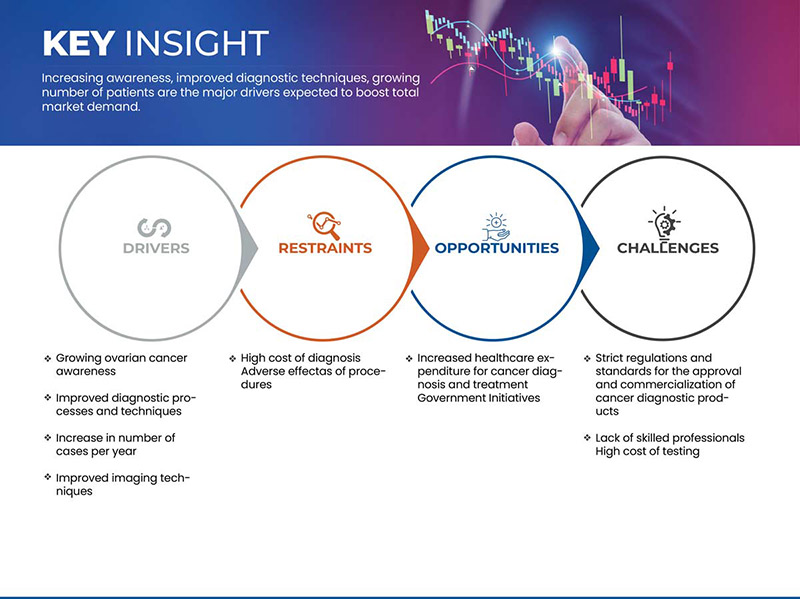
Драйверы
- Растущая осведомленность о раке яичников
Рост осведомленности о раке яичников привел к увеличению спроса на своевременное выявление рака, что привело к росту рынка.
Рак яичников является одной из основных причин роста смертности среди женского населения во всем мире, что подпитывает рост рынка в течение следующих пяти лет. Рак яичников и кист становится все более распространенным из-за различных факторов, таких как факторы окружающей среды и генетические мутации.
Рак яичников — это тип рака, который поражает женские органы, производящие яйцеклетки, яичники. Рак яичников трудно диагностировать, поскольку симптомы неопределенны и часто обнаруживаются только после того, как рак распространился на желудок и таз, что затрудняет его лечение.
В результате, для определения стадии рака, требующей лечения, требуются улучшенные диагностические процессы и методы. Кроме того, растущий уровень смертности от рака яичников вызывает беспокойство, подчеркивая важность раннего выявления, чтобы можно было обеспечить лечение.
Ожидается, что рост осведомленности о раке яичников станет движущим фактором роста рынка.
- Улучшенные диагностические процессы и методы
Скрининговые тесты и обследования используются для выявления заболевания, например рака, у людей, у которых нет никаких симптомов. Было проведено много исследований по разработке скринингового теста на рак яичников, но пока что они не увенчались большим успехом. Два теста, которые чаще всего используются (в дополнение к полному тазовому осмотру) для скрининга рака яичников, — это трансвагинальное ультразвуковое исследование (ТВУЗИ) и анализ крови на CA-125.
TVUS — это тест, который использует звуковые волны для осмотра матки, фаллопиевых труб и яичников, вводя ультразвуковой зонд во влагалище. Он может помочь обнаружить массу (опухоль) в яичнике, но не может фактически сказать, является ли масса раком или доброкачественной. Когда он используется для скрининга, большинство обнаруженных масс не являются раком.
Анализ крови на CA-125 измеряет количество белка CA-125 в крови. У многих женщин с раком яичников наблюдается высокий уровень CA-125. Этот тест может быть полезен в качестве опухолевого маркера, помогающего определить лечение у женщин с известным раком яичников, поскольку высокий уровень часто снижается, если лечение работает. Но проверка уровня CA-125 не так полезна, как скрининговый тест на рак яичников.
Таким образом, ожидается, что благодаря росту числа усовершенствованных диагностических процессов и методов это станет движущим фактором роста рынка.
ОГРАНИЧЕНИЯ
Высокая стоимость диагностики
Во всем мире расходы на лечение рака возросли. Отрасли здравоохранения сталкиваются с рядом проблем, таких как медицинские расходы на лечение рака. Расходы на лечение рака в 2010 году составили 124,60 млрд долларов США, и, по прогнозам, к 2020 году они возрастут до 173,00 млрд долларов США, причем основными факторами являются цены на противораковые препараты и острая больничная помощь. Таким образом, возросшая стоимость производства диагностических препаратов сдерживает рост рынка.
Нехватка квалифицированных специалистов
Медицинские специалисты, участвующие в диагностическом процессе, обязаны и несут этическую ответственность за использование навыков клинического мышления, оценку и решение медицинских проблем пациента. Когда диагноз точный и поставлен своевременно, у пациента есть наилучшая возможность для положительного результата в отношении здоровья, поскольку принятие клинических решений будет адаптировано к правильному пониманию проблемы со здоровьем пациента. Нехватка квалифицированных специалистов может затруднить процесс выздоровления пациента и, таким образом, может помешать росту рынка.
ВОЗМОЖНОСТИ
Увеличение расходов на здравоохранение для диагностики и лечения рака
Во всем мире деятельность в области НИОКР растет из-за расходов на здравоохранение с экономическими показателями, в то время как отрасль здравоохранения занимает второе место среди всех отраслей по объему расходов на здравоохранение. Рост расходов на здравоохранение может привести к лучшему предоставлению возможностей для НИОКР. Ожидается, что спрос на диагностику рака яичников резко возрастет.
Увеличение расходов на здравоохранение для лечения рака также помогает пациентам проходить беспроблемную расширенную диагностику и лечение для быстрого выздоровления. Расходы на здравоохранение состоят из комбинации выплат из собственного кармана (люди платят за свое лечение), государственных расходов и источников, включая медицинское страхование и деятельность неправительственных организаций (НПО). Благодаря этим растущим расходам на здравоохранение для лечения рака это становится возможностью для роста рынка.
ВЫЗОВЫ
Строгие правила и стандарты для одобрения и коммерциализации продуктов диагностики рака
Строгие правила коммерциализации любого продукта на рынке оказываются большой проблемой для производителей диагностических продуктов для рака в США и Европейском регионе. Каждая страна имеет свои собственные правила и имеет свой орган для регулирующих процедур.
В США производители требуют одобрения разрешения на продажу продукции IVD для использования человеком. Продукция должна быть маркирована в соответствии с правилами маркировки. Учреждения, занимающиеся производством и распространением медицинских устройств, предназначенных для коммерческого распространения в США, обязаны зарегистрироваться в FDA. Регистрация предоставляет FDA местонахождение предприятий по производству медицинских устройств и импортеров. Регистрация учреждения не является одобрением учреждения или его устройств FDA, то есть она не предоставляет FDA разрешение на продажу устройства. Если нет исключений, требуется предварительное разрешение на продажу, прежде чем устройство может быть размещено в коммерческом распространении в США.
Нормативные требования к одобрениям маркетинга, а также декларации соответствия и время, необходимое для нормативного обзора, могут различаться для разных продуктов. Компания, которая не получает нормативного одобрения, наносит вред бизнесу, поскольку без получения одобрения на продукцию производители не могут вывести свою продукцию на рынок, и по этой причине строгие правила и стандарты по одобрению и коммерциализации диагностических продуктов для рака выступают в качестве сдерживающего фактора для роста рынка.
Последние события
- В ноябре 2022 года Myriad genetics Inc. объявила о приобретении Gateway Genomics, LLC. Это приобретение усиливает портфель продуктов Myriad Genetics для женского здоровья, расширяя доступ к персонализированным генетическим тестам на репродуктивном этапе жизни женщины и после него. Благодаря SneakPeek Myriad теперь обслуживает женщин на ранних стадиях беременности, предоставляя основанные на данных генетические идеи на протяжении всей их жизни с помощью неинвазивного пренатального скрининга Prequel, скрининга носителей Foresight и теста на наследственный рак MyRisk с оценкой риска для всех предков, это поможет компании увеличить свой доход.
- В октябре 2022 года Quest Diagnostics объявила о новом этапе сотрудничества с Decode health. На начальном этапе сотрудничества обе стороны разработали возможности секвенирования РНК (транскриптома) на основе секвенирования следующего поколения, аналитики и клинического опыта обеих сторон. Сотрудничество имеет важное значение, поскольку данные на основе биомаркеров могут помочь сократить время и стоимость разработки новых диагностических тестов и определить новые лекарственные мишени для различных типов рака (рак молочной железы, простаты и яичников). Это сотрудничество помогает компании находить инновационные пути в области НИОКР и расширяет глобальное присутствие компании.
Масштаб мирового рынка диагностики рака яичников
Глобальный рынок диагностики рака яичников сегментирован по типу продукта, типу процедуры, типу рака и конечному пользователю. Рост среди этих сегментов поможет вам проанализировать сегменты с незначительным ростом в отраслях и предоставить пользователям ценный обзор рынка и рыночные идеи для принятия стратегических решений по определению основных рыночных приложений.
Тип продукта
- Инструменты
- Наборы и реагенты
По типу продукции мировой рынок средств диагностики рака яичников сегментируется на инструменты, наборы и реагенты.
Тип процедуры
- Тестирование маркеров крови
- Медицинский визуальный тест
- Биопсийные тесты
- Генетическое Тестирование
На основе типа процедуры мировой рынок диагностики рака яичников сегментирован на тестирование маркеров крови, медицинские визуализационные тесты, биопсийные тесты и генетическое тестирование.
Тип рака
- Эпителиальная опухоль
- Зародышевая клетка
- Стромально-клеточная опухоль
В зависимости от типа рака мировой рынок диагностики рака яичников сегментирован на эпителиальные опухоли, опухоли герминогенных клеток и опухоли стромальных клеток.
Конечный пользователь
- Центры диагностики рака
- Больничные лаборатории
- Научно-исследовательские институты
- Другие
По признаку конечного пользователя мировой рынок диагностики рака яичников сегментирован на центры диагностики рака, больничные лаборатории, научно-исследовательские институты и другие.
Региональный анализ/информация о мировом рынке диагностики рака яичников
Проведен анализ мирового рынка диагностики рака яичников, а также предоставлены сведения о размерах рынка и тенденциях по странам, типам продуктов, типам процедур, типам рака и конечным пользователям, как указано выше.
В данном отчете о рынке рассматриваются следующие страны: США, Канада и Мексика, Германия, Франция, Великобритания, Италия, Испания, Нидерланды, Россия, Швейцария, Турция, Бельгия и остальные страны Европы, Китай, Япония, Индия, Южная Корея, Австралия, Сингапур, Таиланд, Малайзия, Индонезия, Филиппины и остальные страны Азиатско-Тихоокеанского региона, Бразилия, Аргентина и остальные страны Южной Америки, Южная Африка, Саудовская Аравия, ОАЭ, Египет, Израиль и остальные страны Ближнего Востока и Африки.
Северная Америка доминирует на мировом рынке диагностики рака яичников с точки зрения доли рынка и доходов и продолжит процветать в течение прогнозируемого периода. Это связано с высокой распространенностью и частотой неврологических расстройств в регионе, а растущие инвестиции в НИОКР и запуск новых продуктов стимулируют рынок
Раздел отчета по странам также содержит отдельные факторы, влияющие на рынок, и изменения в регулировании рынка, которые влияют на текущие и будущие тенденции рынка. Такие данные, как новые и заменяющие продажи, демография страны, эпидемиология заболеваний и импортно-экспортные тарифы, являются одними из основных указателей, используемых для прогнозирования рыночного сценария для отдельных стран. Кроме того, при предоставлении прогнозного анализа данных по стране учитываются наличие и доступность глобальных брендов и их проблемы, связанные с конкуренцией со стороны местных и отечественных брендов, а также влияние каналов продаж.
Анализ конкурентной среды и доли мирового рынка диагностики рака яичников
Конкурентная среда рынка диагностики рака яичников содержит сведения по конкурентам. Включены сведения о компании, финансах компании, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, глобальном присутствии, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта, доминировании приложений. Приведенные выше данные касаются только фокуса компаний, связанного с рынком диагностики рака яичников.
Некоторые из основных игроков, работающих на мировом рынке диагностики рака яичников, включают F. Hoffmann-La Roche Ltd, Tosoh India Pvt. Ltd., Luminex Corporation, Quest Diagnostics Incorporated, Thermo Fisher Scientific Inc., Ngenebio, Abbott, Siemens healthcare private limited, Myriad genetics Inc., Bio-rad laboratorys, Inc., R&d systems, Inc., Foundation medicine, Inc., Biosupply ltd, Lcm genect srl, Inex innovate private limited, Abcam plc., Monobind Inc., Fujirebio, Mp biomedicals, Biovision Inc., Boster biology technology, Biogenix Inc. Pvt. Ltd., Genway biotech и Lifespan biosciences, Inc и другие.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Содержание
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GROWTH STRATEGIES ADOPTED BY KEY MARKET PLAYERS
5 INDUSTRY INSIGHTS
5.1 CONCLUSION
6 REGULATIONS OF THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING OVARIAN CANCER AWARENESS
7.1.2 IMPROVED DIAGNOSTIC PROCESSES AND TECHNIQUES
7.1.3 INCREASE IN NUMBER OF NEW CASES EVERY YEAR
7.1.4 IMPROVED IMAGING TECHNIQUES
7.2 RESTRAINS
7.2.1 HIGH COST OF DIAGNOSIS
7.2.2 ADVERSE EFFECTS OF THE TREATMENT
7.3 OPPORTUNITIES
7.3.1 INCREASING HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.3.2 GOVERNMENT INITIATIVES TOWARDS CANCER DIAGNOSTICS
7.4 CHALLENGES
7.4.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
7.4.2 LACK OF SKILLED PROFESSIONALS
8 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.3 KITS AND REAGENTS
9 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE
9.1 OVERVIEW
9.2 BLOOD MARKERS TESTING
9.3 MEDICAL IMAGING TEST
9.4 BIOPSY TEST
9.5 GENETIC TESTING
10 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 EPITHELIAL TUMOR
10.3 GERM CELL
10.4 STROMAL CELL TUMOR
11 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER
11.1 OVERVIEW
11.2 CANCER DIAGNOSTIC CENTERS
11.3 HOSPITAL LABORATORIES
11.4 RESEARCH INSTITUTES
11.5 OTHERS
12 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY GEOGRAPHY
12.1 OVERVIEW
12.2 NORTH AMERICA
12.2.1 U.S.
12.2.2 CANADA
12.2.3 MEXICO
12.3 EUROPE
12.3.1 GERMANY
12.3.2 FRANCE
12.3.3 U.K.
12.3.4 ITALY
12.3.5 SPAIN
12.3.6 RUSSIA
12.3.7 TURKEY
12.3.8 BELGIUM
12.3.9 NETHERLANDS
12.3.10 SWITZERLAND
12.3.11 REST OF EUROPE
12.4 ASIA-PACIFIC
12.4.1 JAPAN
12.4.2 CHINA
12.4.3 SOUTH KOREA
12.4.4 INDIA
12.4.5 AUSTRALIA
12.4.6 SINGAPORE
12.4.7 THAILAND
12.4.8 MALAYSIA
12.4.9 INDONESIA
12.4.10 PHILIPPINES
12.4.11 REST OF ASIA-PACIFIC
12.5 SOUTH AMERICA
12.5.1 BRAZIL
12.5.2 ARGENTINA
12.5.3 REST OF SOUTH AMERICA
12.6 MIDDLE EAST AND AFRICA
12.6.1 SOUTH AFRICA
12.6.2 SAUDI ARABIA
12.6.3 U.A.E
12.6.4 EGYPT
12.6.5 ISRAEL
12.6.6 REST OF MIDDLE EAST AND AFRICA
13 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
13.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
13.3 COMPANY SHARE ANALYSIS: EUROPE
13.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 F. HOFFMANN-LA ROCHE LTD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 TOSOH INDIA PVT. LTD.
15.2.1 COMPANY SNAPSHOT
15.2.2 COMPANY SHARE ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION (2022)
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 QUEST DIAGNOSTICS INCORPORATED (2022)
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 THERMO FISHER SCIENTIFIC INC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 ABCAM PLC (2022)
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 1.7.4 RECENT DEVELOPMENT
15.8 BIOSUPPLY LTD
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 BIO-RADBIO LABORATORIES
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOVISION INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 BIOGENIX INC. PVT. LTD.
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 BOSTER BIOLOGICAL TECHNOLOGY
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.13 FOUNDATION MEDICINE
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 FUJIREBIO
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 GENWAY BIOTECH
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 INEX INNOVATIVE PRIVATE LIMITED
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 LCM GENETIC SRL
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 LIFESPAN BIOSCIENCES, INC
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 MP BIOMEDICALS
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENT
15.2 MONOBIND INC.
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
15.21 MYRIAD GENETICS, INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 REVENUE ANALYSIS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
15.22 NGENEBIO
15.22.1 COMPANY SNAPSHOT
15.22.2 PRODUCT PORTFOLIO
15.22.3 RECENT DEVELOPMENTS
15.23 R&D SYSTEMS, INC.
15.23.1 COMPANY SNAPSHOT
15.23.2 PRODUCT PORTFOLIO
15.23.3 RECENT DEVELOPMENT
15.24 SIEMENS MEDICAL SOLUTIONS
15.24.1 COMPANY SNAPSHOT
15.24.2 REVENUE ANALYSIS
15.24.3 PRODUCT PORTFOLIO
15.24.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Список таблиц
TABLE 1 24-MONTH EPISODE-OF-CARE COSTS FOR EARLY-STAGE AND LATE-STAGE CANCERS BY PAYER (USD BILLION)
TABLE 2 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 GLOBAL INSTRUMENTS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 GLOBAL KITS AND REAGENTS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 6 GLOBAL BLOOD MARKERS TESTING IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 GLOBAL MEDICAL IMAGING TEST IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 GLOBAL BIOPSY TEST IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 GLOBAL GENETIC TESTING IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 11 GLOBAL EPITHELIAL TUMOR IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 GLOBAL GERM CELL IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 GLOBAL STROMAL CELL TUMOR IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 15 GLOBAL CANCER DIAGNOSTIC CENTERS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 GLOBAL HOSPITAL LABORATORIES IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 GLOBAL RESEARCH INSTITUTES IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 GLOBAL OTHERS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 25 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 27 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 28 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 29 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 30 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 31 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 32 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 33 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 34 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 35 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 36 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 37 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 38 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 39 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 40 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 41 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 42 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 43 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 44 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 45 GERMANY OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 47 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 48 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 49 FRANCE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 50 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 52 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 53 U.K. OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 54 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 56 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 57 ITALY OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 58 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 60 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 61 SPAIN OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 62 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 64 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 65 RUSSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 66 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 68 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 69 TURKEY OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 70 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 72 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 73 BELGIUM OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 74 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 76 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 77 NETHERLANDS OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 78 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 80 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 81 SWITZERLAND OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 82 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 84 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 85 REST OF EUROPE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 86 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 87 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 88 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 89 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 90 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 91 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 92 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 93 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 94 JAPAN OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 95 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 97 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 98 CHINA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 99 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 101 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 102 SOUTH KOREA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 103 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 104 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 105 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 106 INDIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 107 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 108 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 109 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 110 AUSTRALIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 112 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 113 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 114 SINGAPORE OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 115 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 117 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 118 THAILAND OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 119 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 121 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 122 MALAYSIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 123 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 124 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 125 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 126 INDONESIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 127 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 128 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 129 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 130 PHILIPPINES OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 131 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 132 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 133 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 134 REST OF ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 135 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 136 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 137 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 138 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 139 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 140 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 141 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 142 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 143 BRAZIL OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 144 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 145 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 146 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 147 ARGENTINA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 148 REST OF SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 149 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 150 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 151 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 152 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 153 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 154 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 155 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 156 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 157 SOUTH AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 158 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 159 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 160 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 161 SAUDI ARABIA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 162 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 163 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 164 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 165 U.A.E OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 166 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 167 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 168 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 169 EGYPT OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 170 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 171 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 172 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 173 ISRAEL OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 174 REST OF MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
Список рисунков
FIGURE 1 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 2 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : DATA TRIANGULATION
FIGURE 3 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : DROC ANALYSIS
FIGURE 4 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : DBMR MARKET POSITION GRID
FIGURE 8 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 11 THE INCREASE IN THE AWARENESS ABOUT OVARIAN CANCER IS EXPECTED TO DRIVE THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2022 TO 2030
FIGURE 12 PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET IN 2022 & 2030
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET , AND ASIA-PACIFI IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 14 EUROPE IS THE FASTEST-GROWING MARKET FOR GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET MANUFACTURERS IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGE OF THE GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET
FIGURE 16 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 17 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 18 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 19 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, 2022
FIGURE 21 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
FIGURE 22 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, CAGR (2023-2030)
FIGURE 23 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, LIFELINE CURVE
FIGURE 24 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 25 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 26 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 27 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 28 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 29 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 30 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 31 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 32 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 33 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : BY COUNTRY (2022)
FIGURE 34 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : BY COUNTRY (2023 & 2030)
FIGURE 35 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 36 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET : BY PRODUCTS (2023-2030)
FIGURE 37 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 38 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 39 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 40 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 41 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 42 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 43 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 44 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 45 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 46 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 47 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 48 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 49 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 50 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 51 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 52 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 53 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 54 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 55 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 56 SOUTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 57 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 58 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 59 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 60 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 61 MIDDLE EAST AND AFRICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 62 GLOBAL OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)
FIGURE 63 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)
FIGURE 64 EUROPE OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)
FIGURE 65 ASIA-PACIFIC OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.