Global Molecular Point Of Care Testing Using Naat Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
37.93 Billion
USD
86.17 Billion
2024
2032
USD
37.93 Billion
USD
86.17 Billion
2024
2032
| 2025 –2032 | |
| USD 37.93 Billion | |
| USD 86.17 Billion | |
|
|
|
|
Сегментация мирового рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) поПродукт (инструменты, расходные материалы и реагенты), Показания (тестирование на респираторные инфекции, тестирование на инфекции, передающиеся половым путем (ИППП), тестирование на инфекции желудочно-кишечного тракта и другие), Конечный пользователь (лаборатории, больницы, клиники, амбулаторные центры, уход на дому, дома престарелых и другие), Способ тестирования (тестирование по рецепту и безрецептурное тестирование), Канал сбыта (больничная аптека, розничная аптека и интернет-аптека) — Тенденции отрасли и прогноз до 2032 года
Объем рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
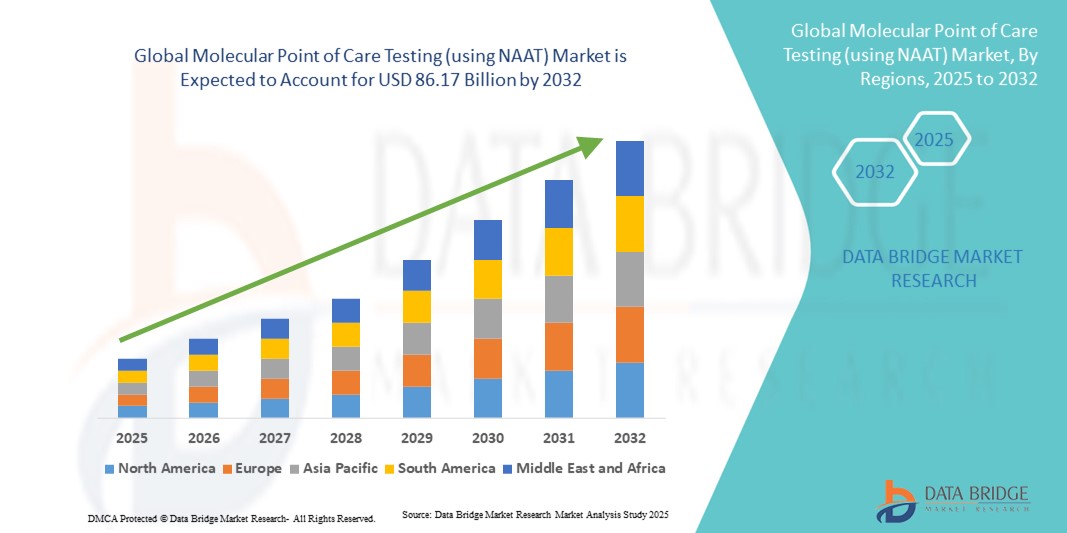
- Объем мирового рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) оценивался в 37,93 млрд долларов США в 2024 году и, как ожидается , достигнет 86,17 млрд долларов США к 2032 году при среднегодовом темпе роста 1,08% в течение прогнозируемого периода.
- Рост рынка обусловлен, прежде всего, ростом распространенности инфекционных заболеваний , спросом на быстрые и точные диагностические тесты и достижениями в области технологий молекулярной диагностики.
- Кроме того, переход к децентрализованному здравоохранению и растущее внедрение мультиплексных тестов способствуют расширению рынка молекулярных POCT-тестов. Эти факторы в совокупности делают молекулярные исследования, проводимые непосредственно в месте оказания медицинской помощи, ключевым компонентом современной диагностики, обеспечивая своевременное и точное выявление различных заболеваний.
Анализ рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
- Молекулярное тестирование на месте оказания медицинской помощи (POCT) с использованием метода амплификации нуклеиновых кислот (NAAT) обеспечивает быстрое и точное выявление инфекционных заболеваний и других медицинских состояний в месте нахождения пациента или рядом с ним, что делает его важнейшим компонентом современной диагностики в больницах, клиниках и децентрализованных медицинских учреждениях.
- Растущее применение молекулярной POCT обусловлено, прежде всего, ростом распространенности инфекционных заболеваний, растущим спросом на своевременную и точную диагностику, а также технологическим прогрессом в области портативных и удобных в использовании устройств для молекулярного тестирования.
- Северная Америка доминировала на рынке молекулярных POCT с наибольшей долей выручки в 39% в 2024 году, чему способствовало раннее внедрение передовых диагностических технологий, высокие расходы на здравоохранение и сильное присутствие ключевых игроков отрасли. В США наблюдался существенный рост благодаря интеграции тестов на основе NAAT в больницы, клиники и пункты неотложной помощи.
- Ожидается, что Азиатско-Тихоокеанский регион станет самым быстрорастущим регионом в прогнозируемый период, что обусловлено ростом инвестиций в инфраструктуру здравоохранения, повышением осведомленности о решениях для быстрой диагностики и растущим спросом на тестирование по месту оказания медицинской помощи в развивающихся экономиках.
- Сегмент тестирования на респираторные инфекции доминировал на рынке молекулярных POCT с долей рынка 43,2% в 2024 году из-за высокой распространенности инфекционных респираторных заболеваний и клинической потребности в быстрой и точной диагностике для определения своевременного лечения и мер по сдерживанию распространения.
Область применения отчета и сегментация рынка молекулярного тестирования в пунктах оказания медицинской помощи (с использованием NAAT)
|
Атрибуты |
Молекулярное тестирование в пунктах оказания медицинской помощи (с использованием NAAT): ключевые аспекты рынка |
|
Охваченные сегменты |
|
|
Страны действия |
Северная Америка
Европа
Азиатско-Тихоокеанский регион
Ближний Восток и Африка
Южная Америка
|
|
Ключевые игроки рынка |
|
|
Рыночные возможности |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, отчеты о рынке, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, анализ цен, анализ доли бренда, опрос потребителей, демографический анализ, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья/расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
Достижения в области быстрого, мультиплексного и ИИ-тестирования
- Значительной и быстрорастущей тенденцией на мировом рынке молекулярных POCT является разработка быстрых мультиплексных устройств NAAT и диагностических платформ на базе искусственного интеллекта, которые повышают скорость, точность и удобство использования тестирования в местах оказания медицинской помощи в больницах, клиниках и децентрализованных учреждениях здравоохранения.
- Например, тест Xpert Xpress SARS-CoV-2/Flu/RSV объединяет обнаружение нескольких патогенов в одном запуске, сокращая время выполнения и оптимизируя рабочий процесс в отделениях для пациентов.
- Интеграция ИИ в молекулярную POCT позволяет реализовать такие функции, как автоматизированная интерпретация результатов тестов, предиктивная аналитика вспышек заболеваний и интеллектуальный контроль качества, повышая достоверность диагностики и снижая количество человеческих ошибок.
- Эти платформы на базе искусственного интеллекта позволяют поставщикам медицинских услуг одновременно управлять несколькими диагностическими параметрами, обеспечивая более быстрые результаты при критических состояниях и снижая зависимость от централизованных лабораторий.
- Эта тенденция к более быстрым, точным и взаимосвязанным решениям для тестирования меняет ожидания от диагностики на месте оказания медицинской помощи. В связи с этим такие компании, как Abbott и Roche, разрабатывают устройства NAAT с поддержкой ИИ, мультиплексными функциями и удобными интерфейсами для медицинского персонала.
- Спрос на молекулярные POCT-устройства, предлагающие мультиплексное обнаружение и функции на основе искусственного интеллекта, стремительно растет как в больницах, так и в амбулаторных условиях, поскольку поставщики медицинских услуг все больше отдают приоритет быстрой и надежной диагностике.
Динамика рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
Водитель
Растущий спрос из-за инфекционных заболеваний и децентрализованного тестирования
- Растущая распространенность инфекционных заболеваний во всем мире в сочетании с переходом к децентрализованному предоставлению медицинской помощи является важным фактором для более широкого внедрения молекулярной POCT с использованием NAAT.
- Например, в 2024 году компания Cepheid запустила расширенные приложения своей платформы GeneXpert, включив в них панели инфекций, передающихся половым путем, и респираторные панели, что отражает усилия по удовлетворению растущих потребностей в диагностике на местах оказания медицинской помощи.
- Поскольку поставщики медицинских услуг стремятся к более быстрой диагностике и своевременному лечению, POCT на основе NAAT обеспечивает высокую чувствительность, специфичность и быстрые результаты, предлагая убедительную альтернативу традиционным централизованным лабораторным исследованиям.
- Кроме того, растущий акцент на контроле вспышек и готовности к чрезвычайным ситуациям делает молекулярную POCT неотъемлемой частью программ по контролю инфекционных заболеваний, позволяя быстро принимать решения о изоляции и лечении.
- Удобство проведения тестирования непосредственно у пациента, минимальные требования к обработке образцов и возможность одновременного проведения нескольких тестов являются ключевыми факторами, способствующими внедрению молекулярной POCT на основе NAAT в больницах, клиниках и полевых условиях.
Сдержанность/Вызов
Нормативные препятствия и эксплуатационные ограничения
- Соблюдение нормативных требований и строгие процедуры утверждения для молекулярных устройств POCT создают значительные трудности для расширения рынка, поскольку платформы на основе NAAT должны соответствовать строгим стандартам точности, безопасности и надежности перед коммерциализацией.
- Например, задержки в одобрении FDA или CE могут замедлить запуск инновационных продуктов POCT, ограничивая доступ к передовым методам диагностики в ситуациях, когда время имеет значение.
- Эксплуатационные ограничения, такие как необходимость в обученном персонале, обслуживании устройств и поставке расходных материалов, могут сдерживать внедрение в условиях ограниченных ресурсов, несмотря на технологические преимущества устройств NAAT.
- Кроме того, относительно высокая стоимость современных мультиплексных или оснащенных искусственным интеллектом устройств POCT по сравнению с традиционными экспресс-тестами может стать препятствием для небольших клиник или регионов с низким уровнем дохода, ограничивая их широкое внедрение.
- Несмотря на постепенное снижение затрат, предполагаемая высокая стоимость сложных молекулярных платформ POCT может препятствовать их внедрению в условиях ограниченного бюджета здравоохранения.
- Преодоление этих проблем посредством оптимизации механизмов регулирования, обучения персонала и разработки экономически эффективных устройств будет иметь решающее значение для устойчивого роста рынка.
Рынок молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
Рынок сегментирован по продукту, показаниям, конечному пользователю, способу тестирования и каналу сбыта.
- По продукту
На основе продукта рынок молекулярных POCT сегментируется на инструменты и расходные материалы и реагенты. Сегмент инструментов доминировал на рынке с наибольшей долей выручки в 55,3% в 2024 году, что обусловлено критической ролью автоматизированных устройств NAAT в получении быстрых и точных результатов. Медицинские учреждения отдают приоритет внедрению инструментов из-за их высокой пропускной способности, надежности и интеграции с лабораторными информационными системами . Инвестиции в современные приборы позволяют больницам и лабораториям оптимизировать рабочие процессы, повышать эффективность и сокращать сроки выполнения диагностических тестов инфекционных заболеваний. Сегмент выигрывает от технологических инноваций, таких как портативные и компактные приборы, подходящие для децентрализованного тестирования. Приборы часто сочетаются с программными платформами для упрощения автоматизированной отчетности и контроля качества. Постоянная техническая поддержка и услуги по обслуживанию, предлагаемые производителями, дополнительно способствуют внедрению.
Ожидается, что сегмент расходных материалов и реагентов продемонстрирует самый быстрый среднегодовой темп роста в 12,5% в период с 2025 по 2032 год, что обусловлено постоянной потребностью в картриджах, тест-наборах и реагентах, используемых в амбулаторных тестах на основе амплификации нуклеиновых кислот (МАНК). Расходные материалы необходимы для ежедневного проведения тестов в больницах, клиниках и лабораториях, обеспечивая поставщикам постоянный доход. Рост осведомленности о тестировании на инфекционные заболевания и увеличение частоты тестирования стимулируют спрос. Мультиплексные анализы, требующие специализированных реагентов, дополнительно ускоряют рост. Технологические инновации, повышающие стабильность реагентов и простоту использования, способствуют их внедрению. Сегмент также выигрывает от улучшения цепочки поставок, что сокращает сроки и стоимость доставки.
- По показаниям
На основе показаний рынок сегментирован на тестирование на респираторные инфекции, тестирование на инфекции, передаваемые половым путем (ИППП), тестирование на инфекции желудочно-кишечного тракта и другие. Сегмент тестирования на респираторные инфекции доминировал на рынке с долей 43,2% в 2024 году из-за высокой распространенности таких патогенов, как SARS-CoV-2, грипп и РСВ. Быстрое и точное выявление респираторных инфекций имеет решающее значение в больницах, клиниках и отделениях неотложной помощи для своевременного лечения и сдерживания распространения заболевания. POCT на основе NAAT обеспечивает высокую чувствительность и специфичность, что делает его предпочтительным методом для респираторных инфекций. Внедрение также стимулируется государственными программами по мониторингу и контролю вспышек заболеваний. Больницы и лаборатории интегрируют эти тесты в рабочие процессы неотложной помощи для быстрого принятия решений. Постоянные инновации в области мультиплексных панелей повышают пропускную способность и эффективность.
Ожидается, что сегмент тестирования на ИППП будет демонстрировать самый быстрый среднегодовой темп роста на уровне 13,1% в период с 2025 по 2032 год, что обусловлено ростом осведомленности, программами скрининга и ростом заболеваемости инфекциями, передаваемыми половым путем. Метод POCT на основе NAAT обеспечивает быстрое, конфиденциальное и точное выявление ИППП, идеально подходящий для использования в клиниках и домашних условиях. Мультиплексные тесты, позволяющие выявлять несколько ИППП в одном тесте, ускоряют внедрение. Расширение возможностей самотестирования и инициатив в области сексуального здоровья повышает спрос. Интеграция с платформами телемедицины улучшает качество отчетности и консультирования. Расширение партнерских отношений между диагностическими компаниями и поставщиками медицинских услуг дополнительно стимулирует рост рынка.
- Конечным пользователем
По типу конечного пользователя рынок молекулярных POCT-тестов сегментируется на лаборатории, больницы, клиники, амбулаторные центры, учреждения по уходу на дому, дома престарелых и другие. Больничный сегмент доминировал на рынке с наибольшей долей выручки в 48,6% в 2024 году благодаря большому количеству пациентов и потребности в быстрой диагностике. Больницы инвестируют в современные устройства NAAT для оптимизации рабочего процесса, контроля вспышек инфекций и поддержки неотложной помощи. Интеграция с больничными информационными системами повышает операционную эффективность. Этот сегмент выигрывает от постоянных технологических инноваций и государственных стимулов. Больницам часто требуются приборы, способные проводить различные типы тестов. Долгосрочные контракты с поставщиками обеспечивают бесперебойные поставки приборов и расходных материалов.
Ожидается, что сегмент услуг по уходу на дому продемонстрирует самый быстрый среднегодовой темп роста на уровне 14,2% в период с 2025 по 2032 год, чему будет способствовать растущий спрос на решения для домашнего тестирования, удобство и конфиденциальность. Наборы МАНК для домашнего использования позволяют пациентам проходить тестирование без посещения клиник или больниц. Самостоятельное тестирование на респираторные инфекции и ИППП способствует его распространению. Интеграция с приложениями для смартфонов обеспечивает простую интерпретацию результатов и телемедицинскую отчетность. Информационные кампании и партнерство с поставщиками услуг телемедицины расширяют охват. Растущее признание домашнего тестирования органами здравоохранения способствует устойчивому росту.
- По способу тестирования
По способу тестирования рынок сегментируется на тестирование по рецепту и безрецептурное тестирование. Сегмент тестирования по рецепту доминировал на рынке с долей 62,3% в 2024 году, что обусловлено нормативными требованиями и профессиональным надзором. Тесты по рецепту обеспечивают точность, надежность и клиническую интерпретацию, особенно в больницах и клиниках. Интеграция с рабочими процессами здравоохранения обеспечивает безопасность пациентов и контроль качества. Они широко внедряются в области высокорисковых инфекционных заболеваний, где требуется экспертное руководство. Устройства часто подключаются к больничным сетям для отслеживания результатов и составления отчетов. Производители продолжают повышать удобство использования, соблюдая нормативные требования.
Ожидается, что сегмент безрецептурных тестов будет демонстрировать самый быстрый среднегодовой темп роста в 13,5% в период с 2025 по 2032 год, что обусловлено растущим спросом на самостоятельное тестирование на респираторные инфекции, ИППП и желудочно-кишечные инфекции. Простые в использовании наборы и интерпретация результатов с помощью смартфона повышают доступность. Потребители предпочитают безрецептурные решения за удобство, конфиденциальность и сокращение количества визитов к врачу. Программы повышения осведомленности и интеграция с телемедициной способствуют внедрению. Розничные продавцы и интернет-аптеки повышают доступность. Этот сегмент выигрывает от использования упрощенных, предварительно упакованных наборов для тестирования, предназначенных для домашнего использования.
- По каналу распространения
По каналам сбыта рынок сегментируется на больничные аптеки, розничные аптеки и интернет-аптеки. Сегмент больничных аптек доминировал на рынке с долей 57,4% в 2024 году, что обусловлено прямыми закупками для стационарных и амбулаторных исследований. Больницы используют аптечные каналы для бесперебойных поставок устройств МАНК, расходных материалов и реагентов. Договоры о оптовых закупках снижают затраты и обеспечивают своевременное пополнение запасов. Интеграция с системами поставок больниц минимизирует дефицит запасов. Больницы часто получают техническую поддержку и обучение по аптечным контрактам. Партнерские отношения с производителями повышают доступность продукции и качество обслуживания.
Ожидается, что сегмент онлайн-аптек продемонстрирует самый быстрый среднегодовой темп роста в 15,1% в период с 2025 по 2032 год, чему будут способствовать удобство заказа, доставка наборов МАНК на дом и растущее внедрение телемедицины. Онлайн-платформы позволяют конфиденциально приобретать как рецептурные, так и безрецептурные тесты. Цифровые маркетинговые кампании повышают осведомленность потребителей. Интеграция с приложениями телемедицины обеспечивает бесперебойную передачу результатов. Потребители всё чаще предпочитают онлайн-доступ из-за удобства и конфиденциальности. Рост электронной коммерции и улучшение логистики способствуют быстрому расширению этого канала сбыта.
Региональный анализ рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
- Северная Америка доминировала на рынке молекулярных POCT с наибольшей долей выручки в 39% в 2024 году, чему способствовало раннее внедрение передовых диагностических технологий, высокие расходы на здравоохранение и сильное присутствие ключевых игроков отрасли. В США наблюдался существенный рост благодаря интеграции тестов на основе NAAT в больницы, клиники и пункты неотложной помощи.
- Поставщики медицинских услуг в регионе отдают приоритет быстрому, точному и надежному тестированию, что делает устройства NAAT для проведения POCT необходимыми для больниц, клиник и отделений неотложной помощи. В частности, в США наблюдается значительный рост благодаря интеграции устройств NAAT в больницы, амбулаторные клиники и тестирование на дому, чему способствуют инновации как от известных диагностических компаний, так и от стартапов.
- Широкое внедрение также поддерживается сильной инфраструктурой здравоохранения, высокими расходами на здравоохранение и технологически развитым населением.
Обзор рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) в США
Рынок молекулярных POCT в США в 2024 году занял наибольшую долю выручки в 79% в Северной Америке, чему способствовало быстрое внедрение передовых диагностических технологий и растущая потребность в своевременном выявлении инфекционных заболеваний. Поставщики медицинских услуг все чаще отдают приоритет тестированию на основе NAAT для респираторных инфекций , ИППП и желудочно-кишечных инфекций из-за его высокой точности и быстроты выполнения. Растущая тенденция к децентрализованному здравоохранению и тестированию на дому дополнительно стимулирует рынок. Более того, интеграция устройств для молекулярных POCT с больничными информационными системами и телемедицинскими платформами вносит значительный вклад в расширение рынка. Сильное присутствие ключевых диагностических компаний и постоянные технологические инновации также способствуют устойчивому росту.
Обзор европейского рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT)
Ожидается, что рынок молекулярной диагностики POCT в Европе будет расти со значительным среднегодовым темпом роста в течение прогнозируемого периода, главным образом за счет растущего спроса на быструю диагностику и роста заболеваемости инфекционными заболеваниями. Государственные инициативы, направленные на продвижение тестирования по месту оказания медицинской помощи и цифровизацию здравоохранения, способствуют его внедрению в больницах, клиниках и лабораториях. Росту также способствуют урбанизация и растущая распространенность инфекций с множественной лекарственной устойчивостью. Европейские поставщики медицинских услуг все чаще внедряют тестирование на основе NAAT для своевременного лечения заболеваний и контроля вспышек. Как частные, так и коммерческие медицинские учреждения внедряют устройства POCT для улучшения качества лечения пациентов. Рынок демонстрирует значительный рост в таких странах, как Германия, Франция и Италия.
Обзор рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) в Великобритании
Ожидается, что рынок молекулярной диагностики по месту жительства (POCT) в Великобритании будет расти значительными среднегодовыми темпами в течение прогнозируемого периода, что обусловлено ростом спроса на быструю диагностику и децентрализованные решения для тестирования. Повышение осведомленности медицинских работников о преимуществах тестирования на основе МАНК, таких как высокая чувствительность и специфичность, стимулирует его внедрение в больницах и клиниках. Государственные инициативы по раннему выявлению и лечению инфекционных заболеваний дополнительно способствуют расширению рынка. Развитая инфраструктура здравоохранения страны и надежные системы электронного здравоохранения улучшают интеграцию устройств и предоставление результатов. Растущее внедрение как в государственном, так и в частном секторе здравоохранения стимулирует рост рынка. Ожидается, что инициативы в области телемедицины и тестирования на дому будут способствовать дальнейшему развитию этой технологии в Великобритании.
Обзор рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) в Германии
Ожидается, что рынок молекулярной POCT-диагностики в Германии будет расти значительными среднегодовыми темпами в течение прогнозируемого периода, чему будет способствовать рост распространенности инфекционных заболеваний и спрос на быструю диагностику. Развитая инфраструктура здравоохранения Германии, значительный акцент на технологические инновации и высокая осведомленность медицинских работников способствуют внедрению POCT-диагностики на основе NAAT. Интеграция устройств POCT с больничными информационными системами и лабораторными сетями повышает эффективность работы. В амбулаторных клиниках и амбулаторных центрах растет предпочтение децентрализованному тестированию. Растет внедрение как в учреждениях здравоохранения, предоставляющих услуги на дому, так и в коммерческих диагностических учреждениях. Постоянные НИОКР и локальное производство инструментов и расходных материалов для POCT дополнительно усиливают рост рынка.
Обзор рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) в Азиатско-Тихоокеанском регионе
Рынок молекулярных POCT-тестов в Азиатско-Тихоокеанском регионе, как ожидается, будет расти самыми быстрыми темпами в 23,5% в течение прогнозируемого периода с 2025 по 2032 год, что обусловлено ростом заболеваемости инфекционными заболеваниями, увеличением инвестиций в здравоохранение и расширением инфраструктуры тестирования в таких странах, как Китай, Япония и Индия. Растущее внимание к децентрализованным и домашним решениям для тестирования в регионе стимулирует их внедрение. Государственные программы, продвигающие цифровое здравоохранение и мониторинг инфекционных заболеваний, способствуют расширению рынка. Присутствие местных производителей обеспечивает доступность устройств и расходных материалов для NAAT. Рост урбанизации, внедрение технологий и повышение осведомленности о ранней диагностике дополнительно стимулируют рост. Спрос в больницах, клиниках и учреждениях по уходу на дому неуклонно растет.
Обзор рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) в Японии
Рынок молекулярных POCT-тестов в Японии набирает обороты благодаря высоким стандартам здравоохранения, внедрению передовых диагностических технологий и растущей осведомлённости об экспресс-тестировании. В стране особое внимание уделяется точному и своевременному выявлению инфекционных заболеваний, что способствует их внедрению в больницах, клиниках и учреждениях домашнего ухода. Интеграция устройств NAAT с больничными сетями и платформами телемедицины повышает эффективность. Старение населения дополнительно увеличивает спрос на удобные и доступные решения для тестирования. Постоянные инновации в области мультиплексных и портативных устройств NAAT поддерживают рынок. Растущий интерес к профилактической медицине и цифровой диагностике ускоряет их внедрение как в бытовом, так и в коммерческом секторе здравоохранения.
Обзор рынка молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) в Индии
В 2024 году рынок молекулярной диагностики POCT в Индии обеспечил наибольшую долю выручки в Азиатско-Тихоокеанском регионе, что объясняется высокой распространенностью инфекционных заболеваний в стране, ростом среднего класса и повышением осведомленности о здравоохранении. Быстрая урбанизация и государственные инициативы по развитию «умного» здравоохранения и цифровой диагностики являются ключевыми драйверами роста. Больницы, клиники и службы ухода на дому все чаще внедряют устройства POCT на основе NAAT для более быстрой диагностики и лечения. Доступные устройства от местных производителей повышают доступность в городских и пригородных районах. Растущий спрос на решения для мультиплексного тестирования еще больше ускоряет внедрение. Эффективные программы общественного здравоохранения и партнерские отношения с диагностическими компаниями продолжают стимулировать рост рынка.
Доля рынка молекулярного тестирования в пунктах оказания медицинской помощи (с использованием NAAT)
Лидерами отрасли молекулярного тестирования на месте оказания медицинской помощи (с использованием NAAT) являются в основном хорошо зарекомендовавшие себя компании, в том числе:
- Thermo Fisher Scientific Inc. (США)
- Hologic, Inc. (США)
- BD (США)
- F. Hoffmann-La Roche Ltd (Швейцария)
- Эбботт (США)
- QIAGEN (Нидерланды)
- БИОМЕРЬЕ (Франция)
- Данахер (США)
- Illumina, Inc. (США)
- Корпорация Sysmex (Япония)
- Siemens Healthineers AG (Германия)
- Seegene Inc. (Южная Корея)
- Guardant Health, Inc. (США)
- Labcorp (США)
- Корпорация точных наук (США)
- 10x Genomics, Inc. (США)
- DNA Genotek Inc. (Канада)
- PathoNostics (Нидерланды)
- Molbio Diagnostics Limited. (Индия)
Каковы последние тенденции на мировом рынке молекулярного тестирования по месту оказания медицинской помощи (с использованием NAAT)?
- В июле 2025 года компания BD (Becton, Dickinson and Company) получила разрешение FDA 510(k) на свою систему BD Veritor™ для SARS-CoV-2, цифрового теста, предназначенного для обнаружения антигенов COVID-19 у лиц с симптомами примерно за 15 минут в местах оказания медицинской помощи, таких как кабинеты врачей, центры неотложной помощи и розничные клиники.
- В октябре 2024 года Всемирная организация здравоохранения (ВОЗ) одобрила первый диагностический тест на mpox (ранее известный как оспа обезьян) для экстренного использования. Это одобрение направлено на расширение глобального доступа к быстрой и точной диагностике mpox, особенно в условиях ограниченных ресурсов. Тест использует технологию амплификации нуклеиновых кислот, что позволяет проводить тестирование непосредственно в медицинском учреждении с высокой чувствительностью и специфичностью.
- В апреле 2024 года компания Roche Diagnostics представила систему cobas® 5800 – платформу автоматизации молекулярной диагностики нового поколения, разработанную для повышения производительности и снижения количества ошибок в лабораториях. Система предлагает стандартизированные анализы и масштабируемые решения, что делает её пригодной для различных объёмов и составов исследований. Повышая уровень автоматизации в молекулярной диагностике, система cobas® 5800 призвана оптимизировать рабочие процессы и обеспечить единообразие результатов в различных условиях тестирования.
- В марте 2023 года компания QuidelOrtho Corporation объявила о получении разрешения от Управления по санитарному надзору за качеством пищевых продуктов и медикаментов США (FDA) на регистрацию нового препарата Sofia® 2 SARS Antigen+ FIA. Sofia 2 SARS Antigen+ FIA — первый экспресс-тест на антиген COVID-19, получивший разрешение FDA на продажу.
- В марте 2023 года тест LumiraDx SARS-CoV-2 Ag был одобрен для использования в пунктах оказания медицинской помощи в учреждениях, работающих в соответствии с Сертификатом об отказе от требований CLIA, Сертификатом соответствия или Сертификатом аккредитации. Этот тест предназначен для использования медицинскими работниками или операторами, имеющими опыт проведения тестов в пунктах оказания медицинской помощи.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.