Europe Lymphangioleiomyomatosis Lam Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
39.90 Million
USD
55.03 Million
2024
2032
USD
39.90 Million
USD
55.03 Million
2024
2032
| 2025 –2032 | |
| USD 39.90 Million | |
| USD 55.03 Million | |
|
|
|
|
Сегментация европейского рынка лимфангиолейомиоматоза (ЛАМ) по типу заболевания (туберозный склероз, сложный ЛАМ и спорадический ЛАМ), типу (диагностика и лечение), осложнениям (пневмоторакс, хилоторакс, опухоль почки, плевральный выпот, отек и скопление жидкости и др.), способу введения (перорально, парентерально и др.), конечному потребителю (больницы, специализированные клиники, диагностические центры, уход на дому и др.), каналу сбыта (прямой тендер, больничные аптеки, розничные аптеки, интернет-аптеки и др.) — тенденции отрасли и прогноз до 2032 г.
Размер европейского рынка лимфангиолейомиоматоза (ЛАМ)
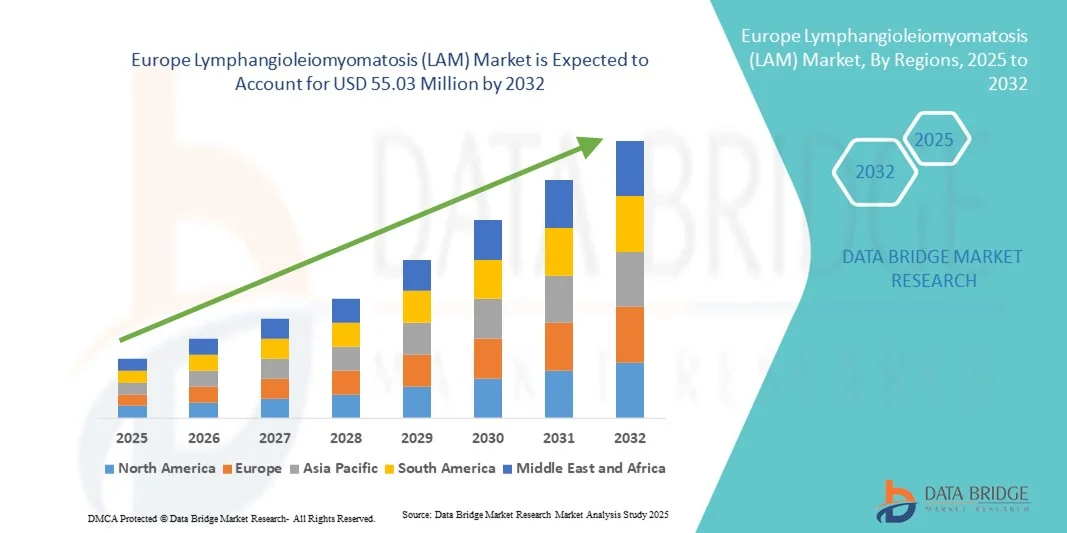
- Объем европейского рынка лимфангиолейомиоматоза (ЛАМ) в 2024 году оценивался в 39,90 млн долларов США и, как ожидается , достигнет 55,03 млн долларов США к 2032 году при среднегодовом темпе роста 4,10% в течение прогнозируемого периода.
- Рост рынка обусловлен в первую очередь повышением осведомленности и показателей диагностики редких заболеваний легких в европейских странах, а также достижениями в области прецизионной медицины и молекулярной диагностики.
- Кроме того, расширение исследовательского сотрудничества, улучшение доступа к орфанным препаратам и расширение клинических испытаний таргетной терапии улучшают результаты лечения и качество ведения пациентов. Сочетание этих факторов ускоряет внедрение инновационных методов лечения с использованием ЛАМ, тем самым усиливая общий рост рынка в регионе.
Анализ европейского рынка лимфангиолейомиоматоза (ЛАМ)
- Лимфангиолейомиоматоз (ЛАМ) – редкое прогрессирующее заболевание легких, поражающее преимущественно женщин. Оно привлекает все больше внимания в Европе благодаря повышению уровня клинической осведомленности, достижениям в исследовании редких заболеваний и усовершенствованным методам диагностики, включающим визуализацию высокого разрешения и генетическое тестирование.
- Растущая потребность в эффективной терапии ЛАМ обусловлена расширением доступа к лечению на основе сиролимуса, ростом инвестиций в программы по редким заболеваниям и совместными исследованиями, поддерживаемыми академическими учреждениями и фармацевтическими компаниями.
- Великобритания доминировала на европейском рынке ЛАМ с наибольшей долей выручки в 35,6% в 2024 году, чему способствовала развитая клиническая инфраструктура, активные организации пациентов, такие как LAM Action, и значительная государственная поддержка разработки орфанных препаратов и реестров редких заболеваний.
- Ожидается, что Германия станет страной с самыми быстрыми темпами роста на европейском рынке ЛАМ в течение прогнозируемого периода, чему будет способствовать увеличение активности клинических исследований, наличие передовых больничных сетей, специализирующихся на легочных заболеваниях, и расширение применения персонализированных подходов к лечению.
- Сегмент «Лечение» доминировал на рынке с наибольшей долей рынка в 62,3% в 2024 году, что было обусловлено растущим внедрением методов лечения, направленных на симптомы ЛАМ и прогрессирование заболевания, а также ростом доступности специализированной помощи в больницах и специализированных клиниках по всей Европе.
Область применения отчета и сегментация европейского рынка лимфангиолейомиоматоза (ЛАМ)
|
Атрибуты |
Ключевые данные о рынке лимфангиолейомиоматоза (ЛАМ) в Европе |
|
Охваченные сегменты |
|
|
Страны действия |
Европа
|
|
Ключевые игроки рынка |
|
|
Рыночные возможности |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативно-правовую базу. |
Тенденции европейского рынка лимфангиолейомиоматоза (ЛАМ)
Растущее внимание к ранней диагностике и повышению осведомленности о редких заболеваниях
- Значительной и набирающей обороты тенденцией на европейском рынке ЛАМ является растущий акцент на ранней диагностике с помощью визуализации высокого разрешения, генетического тестирования и регистров пациентов, что позволяет проводить своевременные вмешательства и улучшать результаты лечения пациентов.
- Например, национальные сети редких заболеваний в Великобритании и Франции внедряют стандартизированные протоколы диагностики ЛАМ, что способствует более раннему выявлению и более эффективному контролю прогрессирования заболевания.
- Достижения в области молекулярной диагностики и идентификации биомаркеров позволяют врачам различать спорадическую ЛАМ и ЛАМ, ассоциированную с туберозным склерозом (ТС), что позволяет оптимизировать персонализированный план лечения.
- Специализированные группы защиты прав пациентов и информационные кампании по всей Европе информируют как медицинских работников, так и пациентов о симптомах ЛАМ, повышая показатели диагностики и сокращая задержки в начале лечения.
- Эта тенденция к более активному скринингу, раннему выявлению и повышению осведомленности о заболеваниях меняет ожидания относительно ухода за пациентами, поскольку компании и научно-исследовательские институты все больше внимания уделяют инновациям в области диагностики.
- Спрос на ранние диагностические решения стремительно растет в больницах и специализированных клиниках, поскольку врачи и пациенты уделяют первостепенное внимание своевременному выявлению ЛАМ для улучшения долгосрочного лечения и качества жизни.
- Расширение сотрудничества между фармацевтическими компаниями и научно-исследовательскими институтами способствует разработке современных диагностических наборов и инструментов прогнозирования для пациентов с ЛАМ. Интеграция цифровых медицинских решений и телемедицины в ведение пациентов с ЛАМ обеспечивает возможность удаленного мониторинга и более быстрых диагностических консультаций, что еще больше ускоряет раннюю диагностику.
Динамика европейского рынка лимфангиолейомиоматоза (ЛАМ)
Водитель
Растущее применение таргетной терапии и орфанных препаратов
- Растущая доступность и внедрение таргетной терапии, включая терапию на основе сиролимуса , является важным фактором развития европейского рынка ЛАМ, улучшая результаты лечения пациентов и стабилизируя функцию легких.
- Например, в марте 2024 года Национальная служба здравоохранения Великобритании расширила доступ к терапии сиролимусом для пациентов со спорадическим ЛАМ, способствуя более широкому внедрению эффективных методов лечения.
- Рост инвестиций в исследования редких заболеваний и программы разработки лекарств для лечения редких заболеваний со стороны как государственных учреждений, так и фармацевтических компаний ускоряет разработку новых методов лечения ЛАМ.
- Повышение осведомленности врачей об ингибиторах mTOR и других целевых методах лечения приводит к тому, что больше пациентов получают терапию, рекомендованную клиническими рекомендациями, что стимулирует рост рынка.
- Улучшенный мониторинг состояния пациентов с помощью специализированных клиник, регистров и телемедицинской поддержки способствует соблюдению режима терапии и долгосрочному ведению заболевания.
- Растущее признание преимуществ таргетной терапии и расширение сетей специализированной медицинской помощи по всей Европе способствуют более широкому внедрению методов лечения ЛАМ в больницах и специализированных клиниках.
- Расширение клинических испытаний и исследований с использованием данных реальной клинической практики в Германии, Франции и Италии способствует одобрению новых методов лечения и повышению уверенности в их эффективности. Партнерство фармацевтических компаний и организаций, защищающих права пациентов с редкими заболеваниями, улучшает программы доступа к лечению и образовательные программы, что дополнительно стимулирует использование лечения.
Сдержанность/Вызов
Ограниченная осведомленность и высокие затраты на лечение
- Ограниченная осведомленность о ЛАМ среди врачей общей практики и некоторых пульмонологов остается ключевой проблемой, что часто приводит к задержке диагностики и начала лечения, что может препятствовать росту рынка.
- Например, исследования, проведенные в Германии и Италии, показывают, что у многих пациентов диагностика занимает несколько лет из-за отсутствия знаний о редких заболеваниях легких среди врачей, работающих на передовой.
- Высокая стоимость сиролимуса и других орфанных препаратов может ограничивать доступ к ним, особенно в странах с бюджетными ограничениями или ограниченным страховым покрытием редких заболеваний.
- Хотя программы поддержки пациентов и государственные субсидии улучшают доступность лечения, финансовое бремя лечения остается препятствием для некоторых пациентов и поставщиков медицинских услуг.
- Обеспечение более широкой осведомленности посредством образовательных кампаний, клинических рекомендаций и обучения врачей имеет решающее значение для улучшения ранней диагностики и использования лечения.
- Для обеспечения более широкого доступа и усиления роста рынка необходимо преодолеть высокие затраты на лечение посредством инициатив по возмещению расходов, программ помощи пациентам и политической поддержки.
- Различия в инфраструктуре здравоохранения в европейских странах могут привести к нестабильному доступу к специализированной помощи и диагностике ЛАМ, ограничивая проникновение на рынок. Проблемы нормативного регулирования, связанные с регистрацией орфанных препаратов и процедурами возмещения стоимости, могут задержать доступность терапии, сдерживая расширение рынка.
Европейский рынок лимфангиолейомиоматоза (ЛАМ)
Рынок сегментирован по типу заболевания, осложнениям, способу введения, конечному потребителю и каналу сбыта.
- По типу заболевания
На основе типа заболевания рынок ЛАМ в Европе сегментируется на ЛАМ с комплексом туберозного склероза (ТСС-ЛАМ), спорадический ЛАМ и другие. Сегмент спорадического ЛАМ доминировал на рынке с наибольшей долей выручки в 58,4% в 2024 году из-за его более высокой распространенности среди женщин репродуктивного возраста. Пациенты со спорадическим ЛАМ нуждаются в постоянном наблюдении и таргетной терапии, что поддерживает спрос на лечение. Обширные клинические исследования, установленные протоколы лечения и активные регистры пациентов по всей Европе способствуют ранней диагностике и вмешательству. Больницы и специализированные клиники все больше внимания уделяют спорадическому ЛАМ из-за его прогрессирующего характера и необходимости терапии ингибиторами mTOR. Такие европейские страны, как Великобритания, Германия и Франция, являются лидерами в принятии лечения и ведении пациентов. Многопрофильная помощь с участием пульмонологов, нефрологов и генетических специалистов еще больше усиливает доминирование сегмента.
Ожидается, что сегмент туберозного склероза с комплексным ЛАМ (TSC-LAM) будет демонстрировать самый быстрый среднегодовой темп роста на уровне 7,9% в период с 2025 по 2032 год, что обусловлено ростом осведомленности о TSC среди генетических консультантов и пульмонологов. Пациентам с TSC-LAM требуется междисциплинарная помощь, включая консультации нефролога и пульмонолога. Улучшенные программы генетического тестирования и ранней диагностики способствуют своевременному началу лечения. Разработка клинических рекомендаций, ориентированных на TSC, и европейских сетей по редким заболеваниям способствует росту. Защита интересов пациентов и образовательные кампании повышают частоту диагностики, способствуя расширению рынка. Продолжающееся сотрудничество в области исследований лечения TSC-LAM привлекает инвестиции в специализированные методы лечения.
- По типу
По типу рынок сегментирован на диагностику и лечение. Сегмент «Лечение» доминировал с долей выручки 62,3% в 2024 году благодаря растущему применению ингибиторов mTOR, таких как сиролимус, для стабилизации функции легких. Больницы и специализированные клиники являются основными каналами назначения лечения, а растущая активность клинических исследований стимулирует его внедрение. Рост осведомленности о прогрессировании заболевания и наличие одобренных орфанных препаратов усиливают спрос. Лечение ЛАМ все чаще интегрируется с программами мониторинга пациентов для обеспечения приверженности лечению. Инфраструктура здравоохранения Европы, особенно Западной Европы, облегчает доступ к передовым методам лечения. Долгосрочная терапия для ведения хронических заболеваний также способствует росту выручки в этом сегменте.
Ожидается, что сегмент диагностики будет демонстрировать самый быстрый среднегодовой рост на 8,1% в период с 2025 по 2032 год благодаря достижениям в области компьютерной томографии высокого разрешения, исследования функции легких и идентификации биомаркеров. Ранняя диагностика ЛАМ критически важна для предотвращения необратимого повреждения легких. Больницы и диагностические центры внедряют генетическое тестирование для дифференциации туберозного склероза и ЛАМ. Европейские инициативы в области редких заболеваний способствуют повышению осведомленности о диагностике и программам обучения врачей. Телемедицина и цифровая отчетность повышают доступность диагностики ЛАМ. Активизация кампаний по защите интересов пациентов и повышению осведомленности о заболевании способствует более быстрому внедрению метода. Увеличение финансирования программ диагностики редких заболеваний также способствует росту сегмента.
- По осложнениям
По характеру осложнений рынок сегментируется на пневмоторакс, хилоторакс, опухоль почки, плевральный выпот, отёк и скопление жидкости и другие. Сегмент пневмоторакса доминировал с долей 41,7% в 2024 году, поскольку рецидивирующий коллапс лёгкого является тяжёлым осложнением, требующим госпитализации и вмешательства. Лечение пневмоторакса часто требует междисциплинарной помощи, что приводит к увеличению стоимости лечения. Специализированные клиники и больницы предоставляют мониторинг и интервенционные процедуры. Осведомлённость пациентов и раннее выявление риска пневмоторакса при ЛАМ растёт по всей Европе. Стандартизированные клинические рекомендации по лечению осложнений поддерживают доминирование этого сегмента. Больницы Германии, Франции и Великобритании лидируют в области расширенного лечения и вмешательства при пневмотораксе.
Ожидается, что сегмент хилоторакса будет демонстрировать самый быстрый рост – среднегодовой темп роста 9,2% в период с 2025 по 2032 год, что обусловлено ростом распространенности лимфатических осложнений у пациентов с ЛАМ. Европейские центры передового опыта в области редких заболеваний легких расширяют спектр услуг по лечению хилоторакса. Ранняя диагностика и малоинвазивные вмешательства способствуют его внедрению. Информационные кампании, освещающие это осложнение, повышают вовлеченность пациентов. Специализированные клиники и больницы инвестируют в передовые методы лечения. Клинические исследования, посвященные лимфатическим осложнениям, способствуют росту сегмента.
- По способу введения
По способу введения рынок сегментирован на пероральные, парентеральные и другие формы. Сегмент пероральных препаратов доминировал с долей выручки 69,1% в 2024 году, что обусловлено широким применением пероральной терапии сиролимусом для длительного лечения. Пероральный прием предпочтителен благодаря удобству, приверженности пациентов лечению и возможности амбулаторного лечения. Больницы, специализированные клиники и службы домашнего ухода облегчают проведение пероральной терапии. Европейская политика возмещения расходов и программы помощи пациентам способствуют внедрению. Пероральный прием легко интегрируется с программами мониторинга для корректировки дозы. Врачи часто предпочитают пероральную терапию из-за ее простоты использования в планах лечения хронических заболеваний.
Ожидается, что сегмент парентеральных препаратов продемонстрирует самый быстрый рост – среднегодовой темп роста 8,5% в период с 2025 по 2032 год. Рост будет обусловлен инъекционными препаратами для лечения запущенных случаев или пациентов с непереносимостью пероральных препаратов. Парентеральное введение обычно применяется в условиях стационара под наблюдением специалистов. Инновации в области биологических препаратов и таргетной терапии способствуют росту. Клинические исследования в Германии, Франции и Италии способствуют расширению рынка. Больничные аптеки и специализированные клиники всё чаще предлагают парентеральные препараты. Телемедицина и программы поддержки пациентов способствуют соблюдению режима лечения.
- Конечным пользователем
По типу конечного пользователя рынок сегментирован на больницы, специализированные клиники, диагностические центры, учреждения, оказывающие медицинскую помощь на дому, и другие. В 2024 году сегмент больниц занимал лидирующие позиции с долей рынка 53,4%, что обусловлено концентрацией специализированных учреждений по лечению и мониторингу ЛАМ. Больницы предоставляют комплексную помощь, включая диагностику, лечение и ведение осложнений. Они служат основными центрами проведения клинических испытаний и исследований редких заболеваний. Европейские больницы все активнее сотрудничают с организациями, защищающими права пациентов, в рамках образовательных программ и программ повышения приверженности лечению. Развитая инфраструктура и многопрофильные модели оказания помощи способствуют доминированию этого сегмента. Больницы остаются предпочтительным выбором для пациентов с тяжелыми или сложными случаями ЛАМ.
Ожидается, что сегмент специализированных клиник продемонстрирует самый быстрый среднегодовой рост на уровне 9,0% в период с 2025 по 2032 год, чему будет способствовать увеличение числа центров, специализирующихся на редких заболеваниях легких. Специализированные клиники предлагают целенаправленную помощь пациентам с ЛАМ, часто обеспечивая долгосрочное наблюдение и персонализированную терапию. Внедрение цифровых медицинских решений и телемедицины расширяет охват населения услугами. Клиники интегрируют междисциплинарные подходы, включая консультации нефролога и генетика в случаях TSC-LAM. Инициативы по повышению осведомленности пациентов стимулируют спрос на услуги специализированных клиник. Сотрудничество с больницами и сетями по редким заболеваниям расширяет возможности клиник и улучшает результаты лечения пациентов.
- По каналу распространения
По каналам сбыта рынок сегментируется на прямые поставки, больничные аптеки, розничные аптеки, интернет-аптеки и другие. Сегмент больничных аптек доминировал с долей выручки 47,8% в 2024 году, поскольку больницы остаются основным местом выдачи ингибиторов mTOR и других препаратов для лечения лейкоэнцефаломиелита (LAM). Больничные аптеки обеспечивают надлежащее хранение, контроль соблюдения режима лечения и консультирование пациентов. Они тесно связаны с клиническими исследованиями и программами лечения. Европейские больничные аптеки расширяют мощности для удовлетворения растущего спроса пациентов. Они также проводят обучение по применению лекарств и профилактике побочных эффектов. Интеграция со специализированными клиниками повышает эффективность дистрибуции и приверженность пациентов лечению.
Ожидается, что сегмент интернет-аптек продемонстрирует самый быстрый среднегодовой рост в 10,3% в период с 2025 по 2032 год, чему будет способствовать более широкое внедрение цифровых технологий, доставка на дом и улучшение доступа к лекарствам для лечения редких заболеваний. Онлайн-платформы обеспечивают удобство для пациентов в отдаленных районах. Интеграция телемедицины с интернет-аптеками повышает приверженность пациентов лечению и качество последующего наблюдения. Обучение пациентов и модели доставки лекарств напрямую пациентам также способствуют их внедрению. Растущая осведомленность о методах лечения редких заболеваний стимулирует онлайн-закупки. Цифровые системы управления рецептами обеспечивают безопасную и своевременную доставку лекарств.
Региональный анализ европейского рынка лимфангиолейомиоматоза (ЛАМ)
- Великобритания доминировала на европейском рынке ЛАМ с наибольшей долей выручки в 35,6% в 2024 году, чему способствовала развитая клиническая инфраструктура, активные организации пациентов, такие как LAM Action, и значительная государственная поддержка разработки орфанных препаратов и реестров редких заболеваний.
- Пациенты и врачи в Великобритании получают доступ к передовым диагностическим возможностям, специализированным лечебным центрам и орфанным препаратам, что в совокупности улучшает контроль заболевания и результаты лечения пациентов с ЛАМ.
- Широкое распространение метода подкрепляется высокой осведомленностью пульмонологов, растущей активностью клинических испытаний и национальными инициативами по редким заболеваниям, что делает Великобританию ключевым центром диагностики и лечения ЛАМ в Европе.
Обзор рынка лимфангиолейомиоматоза (ЛАМ) в Великобритании
Великобритания доминировала на европейском рынке ЛАМ с наибольшей долей выручки в 35,6% в 2024 году благодаря хорошо развитой инфраструктуре здравоохранения, мощной государственной поддержке программ по редким заболеваниям и активным сетям защиты прав пациентов, таким как LAM Action. Больницы и специализированные клиники Великобритании располагают передовыми диагностическими возможностями, включая визуализацию высокого разрешения и генетическое тестирование, что позволяет выявлять заболевания на ранней стадии и эффективно их лечить. Широкая доступность терапии сиролимусом и других целевых методов лечения способствует высокому уровню внедрения. Национальные инициативы и программы регистрации редких заболеваний способствуют своевременной диагностике и долгосрочному наблюдению за пациентами.
Обзор рынка лимфангиолейомиоматоза (ЛАМ) в Германии
Ожидается, что рынок ЛАМ в Германии будет расти значительными среднегодовыми темпами, чему способствуют растущая осведомленность о редких заболеваниях легких, развитая инфраструктура здравоохранения и увеличение активности клинических исследований. Немецкие больницы и специализированные клиники предоставляют комплексные диагностические и лечебные услуги, поддерживая пациентов со спорадическим и туберозным склерозом ЛАМ. Внедрение ингибиторов mTOR и междисциплинарное ведение пациентов, включающее пульмонологию, нефрологию и генетику, способствует росту рынка. Исследовательские инициативы и государственная поддержка программ лечения редких заболеваний расширяют доступ к терапии. Обучение пациентов и мониторинг на основе регистров способствуют ранней диагностике и приверженности лечению. Рынок выигрывает от эффективной политики возмещения расходов и хорошо налаженных сетей специалистов по редким заболеваниям.
Обзор рынка лимфангиолейомиоматоза (ЛАМ) во Франции
Рынок ЛАМ во Франции растёт благодаря наличию специализированных центров ЛАМ, доступу к передовым методам лечения и повышению осведомлённости пациентов. Больницы и диагностические центры внедряют методы визуализации высокого разрешения и генетического тестирования для раннего выявления ЛАМ. Совместные исследования и Европейские референтные сети по редким заболеваниям лёгких улучшают результаты лечения пациентов. Государственная поддержка политики в области редких заболеваний и возмещения расходов облегчает доступ к орфанным препаратам. Клинические испытания и программы поддержки пациентов способствуют более широкому использованию лечения. Информационные кампании, ориентированные на врачей и пациентов, повышают показатели раннего вмешательства и способствуют общему расширению рынка.
Обзор рынка лимфангиолейомиоматоза (ЛАМ) в Италии
Ожидается, что рынок ЛАМ в Италии будет стабильно расти, чему будет способствовать расширение инфраструктуры здравоохранения, регистров пациентов и повышение доступности орфанных препаратов. Больницы и специализированные клиники уделяют особое внимание ранней диагностике и долгосрочному лечению пациентов с ЛАМ. Таргетная терапия, особенно сиролимус, получает всё большее распространение благодаря доказанной эффективности в стабилизации функции лёгких. Итальянские центры активно участвуют в исследовательских сетях по редким заболеваниям, способствуя доступности лечения. Информационные кампании и обучение пульмонологов сокращают задержки в постановке диагноза. Телемедицина и цифровой мониторинг пациентов повышают приверженность лечению и способствуют последующему наблюдению.
Доля европейского рынка лимфангиолейомиоматоза (ЛАМ)
Лидерами европейской отрасли лечения лимфангиолейомиоматоза (ЛАМ) являются в основном хорошо зарекомендовавшие себя компании, в том числе:
- Pfizer Inc. (США)
- Новартис АГ (Швейцария)
- Takeda Pharmaceutical Company Limited (Япония)
- Johnson & Johnson Services, Inc. (США)
- GSK plc (Великобритания)
- АстраЗенека (Великобритания)
- Dr. Reddy's Laboratories Ltd (Индия)
- Zydus Pharmaceuticals, Inc. (Индия)
- Intas Pharmaceuticals Ltd (Индия)
- Apotex Inc. (Канада)
- Amneal Pharmaceuticals LLC (США)
- Корпорация Терумо (Япония)
- Hikma Pharmaceuticals plc (Великобритания)
- TransMedics, Inc. (США)
- XVIVO Perfusion AB (Швеция)
- Taj Pharmaceuticals Limited (Индия)
- Morgan Scientific Inc. (США)
- Sun Pharmaceutical Industries Ltd (Индия)
- Санофи (Франция)
- Берингер Ингельхайм Интернешнл ГмбХ (Германия)
Каковы последние тенденции на европейском рынке лимфангиолейомиоматоза (ЛАМ)?
- В марте 2025 года Фонд ЛАМ представил новый исследовательский проект: «Изучение взаимодействий фибробластов и эндотелия в патогенезе ЛАМ с использованием 3D-моделей сфероидов и пространственной транскриптомики», что свидетельствует о растущем европейском сотрудничестве в области передовых молекулярных исследований и трансляционных моделей, направленных на разработку новых терапевтических подходов к лечению ЛАМ.
- В январе 2025 года в исследовании «Характеристики и результаты лечения пациентов с ЛАМ», опубликованном в журнале Frontiers in Medicine, был проведен анализ когорты европейских пациентов и предоставлены обновленные данные о прогрессировании заболевания, охвате лечением и результатах, что подтверждает необходимость улучшения мониторинга и создания специализированных центров в Европе.
- В апреле 2024 года исследование, опубликованное в журнале European Radiology, продемонстрировало, что протокол КТ грудной клетки с ультранизкой дозой облучения, использующий серебряный фильтр и реконструкцию с глубоким обучением, может снизить дозу облучения примерно на 85%, сохраняя при этом диагностическую точность оценки кист ЛАМ. Это важное достижение для более безопасного рутинного мониторинга в Европе.
- В феврале 2024 года Альянс по лимфангиоматозу и болезни Горхэма опубликовал отчет под названием «Путешествие в сложный мир лимфатических аномалий: прорывы в исследованиях заболеваний легких», в котором были представлены новые сведения о связи ЛАМ с лимфатическими заболеваниями, картографированы генетические мутации в гене TSC2 и описано, как исследования ЛАМ в Европе теперь предлагают более широкие возможности для лечения сложных лимфатических аномалий.
- В мае 2021 года долгосрочное исследование результатов, проведенное в Испании и опубликованное в Scientific Reports, показало, что более половины пациентов с ЛАМ, получавших лечение сиролимусом, сохранили положительный ответ в течение пяти лет, что стало одним из крупнейших европейских исследований в условиях реальной клинической практики и подтверждает необходимость дальнейшего использования терапии ингибиторами mTOR при лечении ЛАМ.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.