Asia Pacific Medical Device Regulatory Affairs Outsourcing Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
8.31 Billion
USD
21.78 Billion
2025
2033
USD
8.31 Billion
USD
21.78 Billion
2025
2033
| 2026 –2033 | |
| USD 8.31 Billion | |
| USD 21.78 Billion | |
|
|
|
|
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Services (Regulatory Affairs Services, Quality Consulting and Medical Writing), Product (Finished Goods, Electronics and Raw Material), Device Type (Class I, Class II and Class III), Application (Cardiology, Diagnostic Imaging, Orthopedic, IVD, Ophthalmic, General and Plastic Surgery, Drug Delivery, Dental, Endoscopy, Diabetes Care and Others), End User (Small Medical Device Company, Medium Medical Device Company and Large Medical Device Company), Country (Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific) Industry Trends and Forecast to 2028
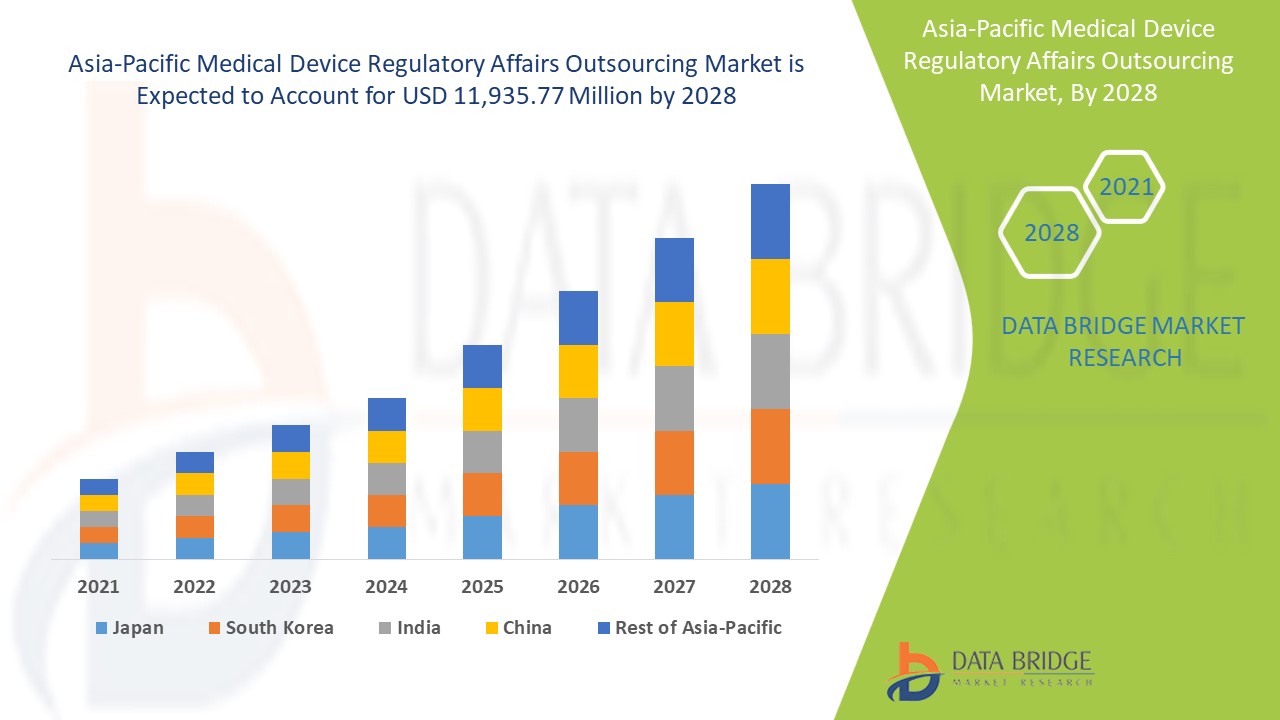 Market Analysis and Insights: Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market
Market Analysis and Insights: Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market
The medical device regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with a CAGR of 12.8% in the forecast period of 2021 to 2028 and is expected to reach USD 11,935.77 million by 2028. The strategic initiative for geographical expansions is anticipated to drive the growth of the medical device regulatory affairs outsourcing market
Outsourcing is an important part of every pharmaceutical and biotechnology companies’ value chain during research and development (R&D). The regulatory affairs outsourcing services entail medical writing and publication of regulatory documentation by professional medical authors, quality control (QC) auditors and publishers who contribute to high-quality clinical research projects. The demand for regulatory services outsourcing has been fueled by a substantial increase in clinical studies conducted in emerging economies, providing a healthy platform for this industry's growth.
The increasing number of patent expirations acts as driver for its growth in the medical device regulatory affairs outsourcing market. The fluctuation in the prices of various medical devices regulatory affairs services acts as restraint for its growth in the medical device regulatory affairs outsourcing market. The awards and recognition provides excellent opportunity for the medical device regulatory affairs outsourcing market growth. The pandemic outbreak of COVID-19 acts as challenge for the growth of the medical device regulatory affairs outsourcing market.
The medical device regulatory affairs outsourcing market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the medical device regulatory affairs outsourcing market scenario contact Data Bridge Market Research for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
 Medical Device Regulatory Affairs Outsourcing Market Scope and Market Size
Medical Device Regulatory Affairs Outsourcing Market Scope and Market Size
The medical device regulatory affairs outsourcing market is segmented on the based on the basis of services, product, device type, application and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of services, the medical device regulatory affairs outsourcing market is segmented into regulatory affairs services, quality consulting and medical writing. In 2021, the regulatory affairs services segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing by key medical device companies.
- On the basis of product, the medical device regulatory affairs outsourcing market is segmented into finished goods, electronics and raw material. In 2021, the finished goods segment is expected to dominate the medical device regulatory affairs outsourcing market due to the increased adoption of regulatory affairs outsourcing for the finished goods by major medical device companies.
- On the basis of device type, the medical device regulatory affairs outsourcing market is segmented into class I, class II and class III. In 2021, the class I segment is expected to dominate the medical device regulatory affairs outsourcing market because of the rising demand for medical devices worldwide to treat patients with chronic diseases.
- On the basis of application, the medical device regulatory affairs outsourcing market is segmented into cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care and others. In 2021, the cardiology segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing for the class III medical devices by key medical device companies.
- On the basis of end user, the medical device regulatory affairs outsourcing market is segmented into small medical device company, medium medical device company and large medical device company. In 2021, the medium medical device company segment is expected to dominate the medical device regulatory affairs outsourcing market due to the rising demand for medical devices worldwide.
Medical Device Regulatory Affairs Outsourcing Market Country Level Analysis
Medical device regulatory affairs outsourcing market is analyzed and market size information is provided by the country, services, product, device type, application and end user as referenced above.
The countries covered in the medical device regulatory affairs outsourcing market report are the Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific.
China is the leading country in the growth of the Asia-Pacific medical device regulatory affairs outsourcing market due to growing R&D activities for the regulatory affairs services segment.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Increasing Geographical Expansion Activities by Medical Device Companies is boosting the Medical Device Regulatory Affairs Outsourcing Market Growth
The medical device regulatory affairs outsourcing market also provides you with detailed market analysis for every country growth in medical device regulatory affairs outsourcing industry. Moreover, it provides detailed information regarding medical device regulatory affairs outsourcing sales, impact of regulatory scenarios and trending parameters regarding medical device regulatory affairs outsourcing market. The data is available for historic period 2011 to 2019.
Competitive Landscape and Medical Device Regulatory Affairs Outsourcing Market Share Analysis
The medical device regulatory affairs outsourcing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to the Asia-Pacific medical device regulatory affairs outsourcing market.
The major companies covered in the Asia-Pacific medical device regulatory affairs outsourcing market report are Parexel International Corporation, North American Science Associates, Inc., SGS SA, Creganna (a subsidiary of TE Connectivity), Intertek Group plc, WuXi AppTec, Charles River Laboratories, Celestica Inc., Freyr, Cactus Communications, Cekindo Business International, Eurofins Scientific, TÜV SÜD, Sterigenics U.S., LLC – A Sotera Health company, TE Connectivity, FLEX LTD., Heraeus Holding, Integer Holdings Corporation, Nortech Systems, Inc., IQVIA, Covance, Plexus Corp., Sanmina Corporation, OMICS International, East West Manufacturing, Jabil Inc., Omron Corporation among other global and domestic players. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Many contracts and agreements are also initiated by the companies’ worldwide which are also accelerating the medical device regulatory affairs outsourcing market.
For instance,
- In January 2020, Charles River Laboratories announced that it has acquired HemaCare Corporation (HemaCare), a company specialized in cell therapy. The company's strategic acquisition has expanded its product portfolio of early-stage research and manufacturing support solutions leading to increased sales and revenue.
Collaboration, product launch, business expansion, award and recognition, joint ventures and other strategies by the market players is enhancing the company footprints in the medical device regulatory affairs outsourcing market which also provides the benefit for organization’s profit growth.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.














