Asia Pacific Anti Nuclear Antibody Test Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
558.77 Million
USD
1,639.25 Million
2024
2032
USD
558.77 Million
USD
1,639.25 Million
2024
2032
| 2025 –2032 | |
| USD 558.77 Million | |
| USD 1,639.25 Million | |
|
|
|
|
Сегментация рынка тестов на антиядерные антитела в Азиатско-Тихоокеанском регионе по типу антител (извлекаемые ядерные антигены (ENA), анти-DSDNA и гистоны, антитела анти-DFS70, анти-PM-SCL, антитела антицентромеры, анти-SP100 и другие), продукту (инструменты, расходные материалы и реагенты, а также услуги), методу (ИФА, непрямая иммунофлуоресценция (НИФ), блоттинг-тест, антигенный микрочип, методы на основе геля, мультиплексный анализ, проточная цитометрия, пассивная гемагглютинация (ПГА) и другие), применению (аутоиммунные заболевания и инфекционные заболевания), по конечному пользователю (больницы, лаборатории, диагностические центры, научно-исследовательские институты и другие), каналу распространения (прямые торги, розничные продажи и сторонние дистрибьюторы и другие) - отраслевые тенденции и прогноз 2032
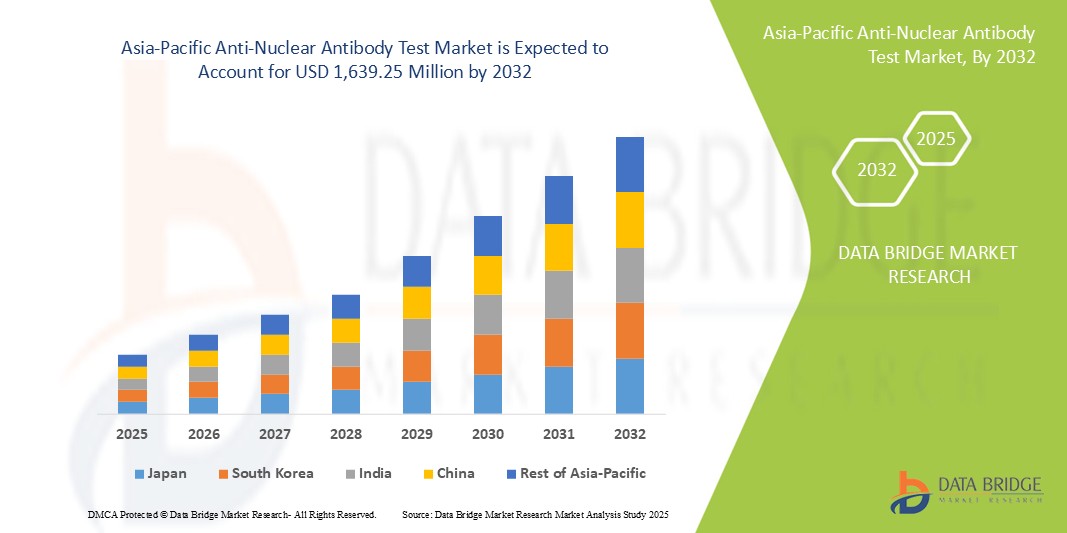
Размер рынка тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе
- Объем рынка тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе оценивался в 558,77 млн долларов США в 2024 году и, как ожидается , достигнет 1 639,25 млн долларов США к 2032 году при среднегодовом темпе роста 14,40% в прогнозируемый период.
- Этот рост обусловлен такими факторами, как рост распространенности аутоиммунных заболеваний, технологические достижения в диагностике и повышение осведомленности в области здравоохранения.
Анализ рынка тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе
- Тесты на антинуклеарные антитела (ANA) являются важнейшими диагностическими инструментами, используемыми для обнаружения аутоантител в крови, помогая в диагностике аутоиммунных заболеваний , таких как системная красная волчанка (СКВ), ревматоидный артрит и синдром Шегрена. Эти тесты играют важную роль в раннем выявлении и лечении этих состояний
- Растущая заболеваемость аутоиммунными заболеваниями, такими как системная красная волчанка (СКВ) и ревматоидный артрит, обусловливает спрос на тестирование АНА во всем регионе.
- Ожидается, что Китай будет доминировать на рынке тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе с долей рынка 32,4%, что обусловлено большой численностью населения, растущей распространенностью аутоиммунных заболеваний и значительными инвестициями в инфраструктуру здравоохранения.
- Ожидается, что Индия станет самой быстрорастущей страной с среднегодовым темпом роста 14,6% на рынке тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе, что будет обусловлено ростом осведомленности о здравоохранении и увеличением расходов на здравоохранение.
- Ожидается, что ИФА (иммуноферментный анализ) будет доминировать на рынке с долей рынка 52,60% благодаря своей высокой чувствительности, экономической эффективности и широкой доступности, что делает его предпочтительным выбором для широкомасштабного скрининга в клинических лабораториях.
Область применения отчета и сегментация рынка тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе
|
Атрибуты |
Ключевые данные о рынке тестов на антиядерные антитела в Азиатско-Тихоокеанском регионе |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Азиатско-Тихоокеанский регион
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают анализ импорта и экспорта, обзор производственных мощностей, анализ потребления продукции, анализ ценовых тенденций, сценарий изменения климата, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья и расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка тестов на антиядерные антитела в Азиатско-Тихоокеанском регионе
«Достижения в тестировании на антинуклеарные антитела (ANA) и автоматизации для диагностики аутоиммунных заболеваний в Азиатско-Тихоокеанском регионе»
- Одной из заметных тенденций в развитии тестирования ANA в Азиатско-Тихоокеанском регионе является все более широкое использование автоматизированных платформ тестирования и усовершенствованных диагностических технологий.
- Эти инновации значительно повышают скорость, точность и эффективность тестирования АНА, обеспечивая более высокую чувствительность и надежность при выявлении аутоиммунных заболеваний.
- Например, автоматизированные системы иммунофлуоресцентного анализа (ИФА) оптимизируют процесс тестирования, позволяя лабораториям обрабатывать большие объемы образцов с меньшим количеством человеческих ошибок и более быстрыми сроками выполнения, что имеет решающее значение для точной диагностики аутоиммунных заболеваний.
- Рост разработки и внедрения устройств для тестирования в точках оказания помощи еще больше способствует росту рынка. Эти устройства обеспечивают более быстрые результаты, облегчая своевременное принятие решений о лечении, особенно в отдаленных районах с ограниченным доступом к специализированной медицинской помощи
- Эти достижения трансформируют аутоиммунную диагностику в Азиатско-Тихоокеанском регионе, повышая точность диагностики и улучшая раннее выявление аутоиммунных заболеваний, тем самым стимулируя спрос на более эффективные и доступные решения для тестирования АНА.
Динамика рынка тестов на антиядерные антитела в Азиатско-Тихоокеанском регионе
Водитель
«Растущая распространенность аутоиммунных заболеваний»
- Растущая распространенность аутоиммунных заболеваний, таких как системная красная волчанка (СКВ), ревматоидный артрит и аутоиммунные заболевания щитовидной железы, значительно увеличивает спрос на тестирование АНА в Азиатско-Тихоокеанском регионе.
- Поскольку население многих стран Азиатско-Тихоокеанского региона стареет, а факторы окружающей среды способствуют изменениям в иммунной системе, наблюдается заметный рост заболеваемости аутоиммунными заболеваниями, особенно среди женщин.
- Поскольку эти заболевания диагностируются у все большего числа людей, растет спрос на тесты на АНА, которые обеспечивают точную диагностику и лучшие результаты лечения заболеваний.
Например,
- В октябре 2023 года Всемирная организация здравоохранения (ВОЗ) сообщила, что распространенность аутоиммунных заболеваний в Азии растет, особенно в таких странах, как Китай и Индия, где наблюдается большое старение населения и растет осведомленность о проблемах здравоохранения.
- В результате рост заболеваемости аутоиммунными заболеваниями создал острую необходимость в тестировании на АНА, тем самым расширяя рынок диагностических инструментов.
Возможность
«Технологические достижения в тестировании ANA»
- Разработка автоматизированных высокопроизводительных диагностических платформ для АНА-тестирования открывает значительные возможности для повышения эффективности и точности тестирования.
- Автоматизация может снизить риск человеческой ошибки, увеличить производительность лабораторий и ускорить процесс диагностики, сделав его более доступным для большего количества людей, особенно в сельских или недостаточно обслуживаемых регионах.
- Кроме того, внедрение тест-устройств для определения АНА в местах оказания медицинской помощи (POC) дает возможность улучшить диагностику в местах с ограниченным доступом к специализированным лабораториям.
Например,
- В марте 2024 года исследование, опубликованное в Asian Journal of Clinical Immunology, показало, что автоматизированные системы ИФА (иммунофлуоресцентного анализа) становятся все более популярными в таких странах, как Япония и Южная Корея, повышая общую точность и эффективность тестирования в загруженных клинических условиях.
- Благодаря использованию автоматизации и технологии POC тесты на АНА можно проводить быстрее, обеспечивая более быстрые результаты диагностики и позволяя своевременно проводить медицинское вмешательство при аутоиммунных заболеваниях.
Сдержанность/Вызов
«Высокая стоимость и ограниченный доступ к диагностической инфраструктуре»
- Стоимость тестирования ANA, а также ограниченная доступность современного диагностического оборудования в сельских или развивающихся районах создают проблему для роста рынка в Азиатско-Тихоокеанском регионе.
- Хотя тесты на АНА имеют решающее значение для диагностики аутоиммунных заболеваний, расходы на поддержание современных диагностических систем могут стать препятствием для поставщиков медицинских услуг в условиях нехватки ресурсов.
- Кроме того, потребность в высококвалифицированных лаборантах и сложный характер тестирования на АНА ограничивают доступ к этим диагностическим услугам в некоторых регионах.
Например,
- В августе 2023 года в статье, опубликованной Индийской медицинской ассоциацией, подчеркивалась проблема доступности и инфраструктуры, особенно в сельских районах Индии, где доступ к передовым диагностическим услугам ограничен как из-за финансовых ограничений, так и из-за нехватки подготовленных специалистов.
- В результате системы здравоохранения в этих регионах могут столкнуться с трудностями в обеспечении своевременного и точного тестирования на АНА, что будет препятствовать общему росту рынка в регионе.
Масштаб рынка тестов на антиядерные антитела в Азиатско-Тихоокеанском регионе
Рынок сегментирован по типу антител, продукту, технологии, применению, конечному пользователю и каналу сбыта.
|
Сегментация |
Субсегментация |
|
По типу антител |
|
|
По продукту |
|
|
По технике |
|
|
По применению
|
|
|
Конечным пользователем |
|
|
По каналу распространения |
|
Ожидается, что в 2025 году ИФА будет доминировать на рынке с наибольшей долей в сегменте технологий.
Ожидается, что сегмент ИФА (иммуноферментный анализ) будет доминировать на рынке тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе с наибольшей долей в 52,60% в 2025 году. Это доминирование обусловлено в первую очередь его высокой чувствительностью, экономической эффективностью и широкой доступностью, что делает его предпочтительным выбором для крупномасштабного скрининга в клинических лабораториях. Надежность и эффективность ИФА в выявлении аутоиммунных заболеваний дополнительно способствуют его лидерству на рынке.
Ожидается, что извлекаемые ядерные антигены (ENA) составят наибольшую долю на рынке типов антител в течение прогнозируемого периода.
Ожидается, что в 2025 году сегмент извлекаемых ядерных антигенов (ENA) будет доминировать на рынке с наибольшей долей в 29,35%. Это доминирование в первую очередь обусловлено критической ролью тестов ENA в диагностике определенных аутоиммунных заболеваний, таких как системная красная волчанка (СКВ) и синдром Шегрена. Точность и специфичность тестирования ENA делают его жизненно важным компонентом аутоиммунной диагностики, что обуславливает его лидерство на рынке.
Анализ рынка тестов на антинуклеарные антитела в Азиатско-Тихоокеанском регионе
- Китай занимает доминирующую долю на рынке тестов ANA в Азиатско-Тихоокеанском регионе с долей рынка 32,4%, что обусловлено большой численностью населения, растущей распространенностью аутоиммунных заболеваний и значительными инвестициями в инфраструктуру здравоохранения.
- Япония — ведущая страна в Азиатско-Тихоокеанском регионе, известная своими передовыми технологиями здравоохранения, высокими расходами на здравоохранение и сильным акцентом на раннее выявление заболеваний. Япония вносит около 20% в региональный рынок тестов ANA, что отражает ее хорошо налаженную систему здравоохранения и стареющее население
- Прогнозируется, что Индия будет демонстрировать самые высокие совокупные годовые темпы роста (CAGR) на рынке с долей рынка в 14,6%, что обусловлено ростом осведомленности о здравоохранении и увеличением расходов на здравоохранение.
- Страны Юго-Восточной Азии, включая Индонезию, Малайзию и Филиппины, переживают быстрый рост рынка из-за увеличения инвестиций в здравоохранение, повышения осведомленности об аутоиммунных заболеваниях и расширения диагностических возможностей.
- В Австралии и Новой Зеландии также наблюдается устойчивый рост рынка, чему способствуют высокие стандарты здравоохранения, широкое распространение страхового покрытия и рост численности пожилого населения.
- Растущая тенденция диагностики в пунктах оказания медицинской помощи (POC) в Азиатско-Тихоокеанском регионе, особенно в отдаленных и сельских регионах, увеличивает спрос на тестирование ANA. Решения для диагностики POC в регионе
Тест на антиядерные антитела в Азиатско-Тихоокеанском регионе
Конкурентная среда рынка содержит сведения о конкурентах. Включены сведения о компании, ее финансах, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, глобальном присутствии, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта, доминировании приложений. Приведенные выше данные касаются только фокуса компаний на рынке.
Основными лидерами рынка, работающими на рынке, являются:
- Thermo Fisher Scientific Inc. (США)
- Bio-Rad Laboratories, Inc. (США)
- Эбботт (США)
- Euroimmun Medizinische Labordiagnostika AG (Германия)
- Revvity Inc. (США)
- Trinity Biotech (Ирландия)
- LIFESPAN BIOSCIENCES, INC (США)
- ORIGENE TECHNOLOGIES, INC. (США)
- Корпорация Abnova (Тайвань)
- CUSABIO TECHNOLOGY LLC (США)
- Biorbyt Ltd. (Англия)
Последние разработки на рынке тестов на антиядерные антитела в Азиатско-Тихоокеанском регионе
- В марте 2025 года компания Shenzhen Mindray Bio-Medical Electronics Co., Ltd. объявила о запуске своей платформы тестирования ANA следующего поколения в Азиатско-Тихоокеанском регионе, разработанной для повышения точности и пропускной способности тестов. Новая система объединяет передовую автоматизацию и искусственный интеллект для предоставления быстрых и надежных результатов диагностики аутоиммунных заболеваний, поддерживая более быстрое принятие клинических решений.
- В феврале 2025 года корпорация Sysmex, ведущая японская диагностическая компания, представила свою усовершенствованную платформу ELISA для тестирования ANA, отличающуюся повышенной чувствительностью и точностью. Эта разработка направлена на удовлетворение растущего спроса на точную аутоиммунную диагностику на рынке Азиатско-Тихоокеанского региона, обусловленного ростом осведомленности об аутоиммунных заболеваниях и расширением инфраструктуры здравоохранения.
- В январе 2025 года компания Bio-Rad Laboratories, Inc. расширила свой портфель тестов ANA в Азиатско-Тихоокеанском регионе, внедрив новую систему мультиплексного анализа, предлагающую комплексное профилирование аутоантител для более точной диагностики заболеваний. Этот запуск соответствует стратегии компании по укреплению своего присутствия на рынке в быстрорастущем секторе диагностики Азиатско-Тихоокеанского региона.
- В декабре 2024 года компания MBL (Medical & Biological Laboratories Co., Ltd.) представила свою новейшую линейку диагностических реагентов для тестирования ANA на рынке Азиатско-Тихоокеанского региона. Эти реагенты предназначены для получения высокоспецифичных и точных результатов, что способствует ранней диагностике и эффективному лечению аутоиммунных заболеваний, таких как системная красная волчанка (СКВ) и ревматоидный артрит.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.