North America Syndromic Multiplex Diagnostic Market - By Product and Services (Reagents & Consumables, Instruments, Software & Accessories and Services), Infection Type (Viral, Bacterial, Parasites and Fungal), Disease (Respiratory Infections, Gastroenteritis, Sexually Transmitted Infections, Sepsis, Meningitis and Others), Panels Type (Respiratory Panel, GI-Enteric Panel, Sexually Transmitted Disease Panel, Blood-Sepsis Panel, Meningitis Panel and Others), End User (Clinical Laboratories, Hospitals, Pharmaceutical & Biotechnology Companies, Research Institutes and Others) Industry Trends and Forecast to 2029.
Market Definition and Insights
Syndromic multiplex diagnostic is a type of advanced diagnostic test utilised to detect infectious diseases such as respiratory infection, infective gastroenteritis, sexually transmitted infections, sepsis, and meningitis, among other types of infectious diseases. The syndromic multiplex diagnostic also helps the clinicians or hospitals to detect the symptoms and signs of the various types of diseases. This lets the health care providers provide the right treatment for the patients and offer more precise outcomes and care that can be performed more quickly.
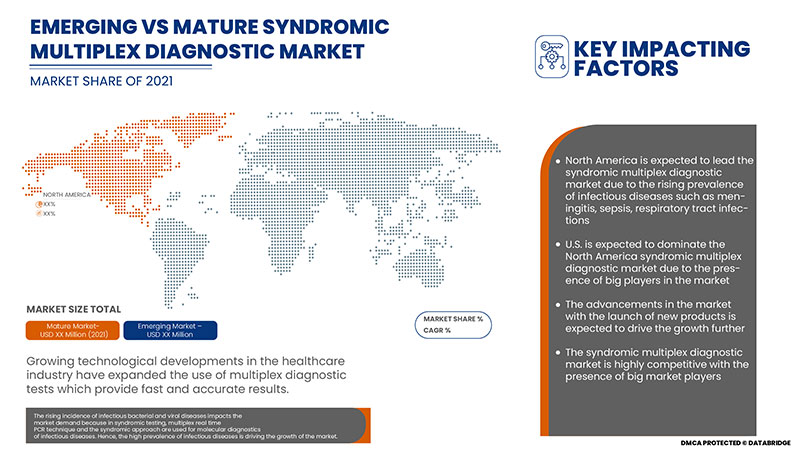
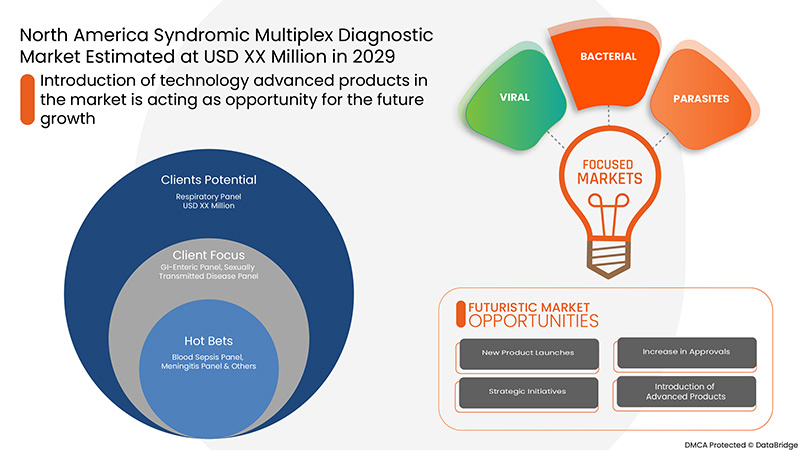
Syndromic multiplex testing is used to diagnose many pathogens simultaneously. In the syndromic multiplex diagnostic, various types of reagents & consumables and instruments & accessories are used, which helps maintain accuracy and provide fast diagnosis results. These multiplex tests are rapidly diagnosed with certain infections, allowing clinical management decisions to be made promptly. The tests based on multiplex technology are known as test panels. The panels used in syndromic testing are designed to diagnose multiple diseases associated with the same or similar syndrome type. These panels help evaluate the cause of the disease at the point of care. Gastrointestinal panels and respiratory panels are the types of syndromic panels.
Syndromic multiplex testing utilizes the advanced technology of multiplex PCR which provides accurate and fast diagnostic results with the help of the multiple panels used in syndromic multiplex diagnostic to provide diagnostic results within an hour. The new generations of syndromic multiplex can rapidly identify the common type of pathogens in the respiratory specimens, blood and cerebrospinal. The use of multiplex panels is associated with quicker turnaround time, reduction of other unnecessary laboratory tests, faster diagnosis and targeted treatment.
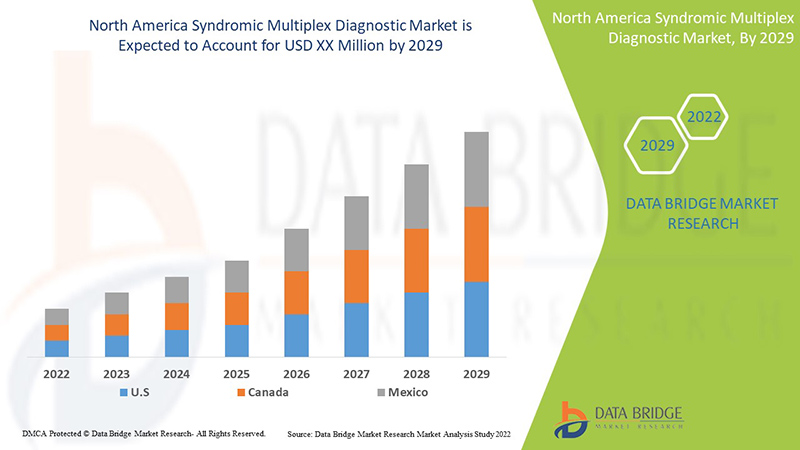
Data Bridge Market Research analyses that the North America syndromic multiplex diagnostic market will grow at a CAGR of 9.0% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
예측 기간 |
2022년부터 2029년까지 |
|
기준 연도 |
2021 |
|
역사적 연도 |
2020 |
|
양적 단위 |
매출은 백만 달러, 볼륨은 단위, 가격은 달러로 표시 |
|
다루는 세그먼트 |
제품 및 서비스(시약 및 소모품, 기기, 소프트웨어 및 액세서리 및 서비스), 감염 유형(바이러스, 박테리아, 기생충 및 진균), 질병(호흡기 감염, 위장염, 성병, 패혈증, 뇌막염 및 기타), 패널 유형(호흡기 패널, GI-장 패널, 성병 패널, 혈액-패혈증 패널 , 뇌막염 패널 및 기타), 최종 사용자(임상 실험실, 병원, 제약 및 생명공학 회사, 연구소 및 기타) |
|
적용 국가 |
미국, 캐나다, 멕시코 |
|
시장 참여자 포함 |
BioFire Diagnostics(bioMérieux SA의 자회사), Seegene Inc.(한국), Luminex Corporation. DiaSorin Company(미국), F. Hoffmann-La Roche Ltd(스위스), BD(미국), Bio-Rad Laboratories, Inc.(미국), Cepheid(Danaher(미국)의 자회사), QIAGEN(독일), Abbott(미국), Hologic, Inc.(미국), Thermo Fisher Scientific Inc.(미국), Siemens Healthcare GmbH(독일), Akonni Biosystems, Inc.(미국), Biocartis(벨기에), QuantuMDx Group Ltd.(영국), Applied BioCode, Inc.(미국), Prominex Inc.(미국), Nanomix, Inc.(미국), Curetis(OpGen, Inc.의 자회사)(독일) |
증후군 멀티플렉스 진단 시장 역학
운전자
- 감염병 유병률 증가
감염성 박테리아 및 바이러스성 질병의 증가는 시장 수요에 영향을 미치는데, 그 이유는 증후군 검사에서 다중 실시간 PCR 기술과 증후군 접근법이 감염성 질병의 분자적 진단에 사용되기 때문입니다.
- 급성 호흡기 증후군 코로나바이러스 검사에 대한 규제 승인 증가
2020년 5월, biomérieux SA는 COVID-19 질병을 유발하는 SARS-CoV-2를 포함하여 호흡기 감염을 일으키는 22가지 병원체를 탐지하는 데 사용되는 BIOFIRE RP2.1 패널에 대한 FDA 긴급 사용 승인(EUA)을 받았습니다.
FDA나 CE 마크와 같은 규제 기관은 SARS-CoV-2 패널의 상용화와 COVID-19 질병과 관련된 바이러스를 검출하기 위한 테스트에 대한 긴급 사용 승인(EUA)을 제공하며, 이는 시장 성장을 견인하는 요인입니다.
기회
- 시장 참여자들이 취한 전략적 이니셔티브
2021년 3월, F. Hoffman-La Roche Ltd는 선도적인 멀티플렉스 분자 진단 공급업체인 GenMark Diagnostics를 인수했습니다. 이 인수를 통해 회사는 Roche의 분자 진단 포트폴리오를 확대할 수 있었습니다. 시장 참여자들이 취한 이러한 전략적 이니셔티브에는 집중된 세그먼트 제품 출시가 포함되며, 이를 통해 글로벌 도달 범위를 확장하고 제품 포트폴리오를 개선하고 시장 성장의 기회로 작용하고 있습니다.
- 기술적으로 진보된 제품의 소개
증후군 멀티플렉스 검사는 멀티플렉스 PCR의 첨단 기술을 활용하여 표적 핵산을 검출, 분리 또는 증폭하여 정확하고 빠른 진단 결과를 제공합니다. 이 첨단 기술은 45~60분 이내에 진단 결과를 제공합니다. 따라서 기술적으로 진보된 제품의 개발은 시장 성장의 기회로 작용하고 있습니다.
제지/도전
- 진단 제품의 높은 비용
멀티플렉스 증후군 검사 애플리케이션은 실시간 중합효소 연쇄 반응(PCR)을 활용하여 증폭 곡선과 정확한 값으로 결과를 제공합니다. 증후군 멀티플렉스 진단에 사용되는 기기는 높은 유지 관리 비용이 필요합니다. 따라서 기기의 높은 비용은 시장에 도전이 됩니다.
COVID-19 이후 증후군 멀티플렉스 진단 시장 에 미치는 영향
COVID-19는 시장에 긍정적인 영향을 미쳤습니다. 시장 참여자들은 SARS-CoV 바이러스를 탐지하기 위한 다양한 제품을 출시하고 있습니다. COVID-19 이후 규제 기관에서 제품에 대한 승인이 증가하여 시장 성장이 증가했습니다.
최근 개발
- 2021년 3월, Diasorin Company인 Luminex Corporation은 확장된 NxTAG 호흡 패널 검사에 대한 FDA 긴급 사용 허가 및 CE 마크를 받았습니다. 이 승인은 회사의 매출을 늘리는 데 도움이 되었습니다.
증후군 멀티플렉스 진단 시장 범위
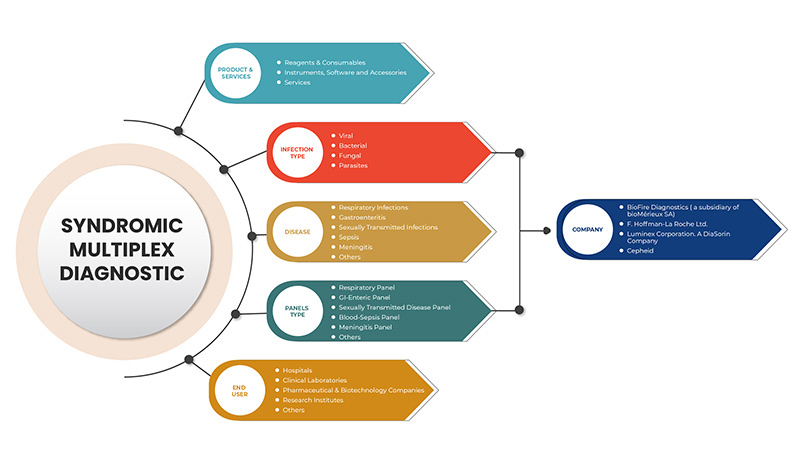
증후군 멀티플렉스 진단 시장은 제품 및 서비스, 감염 유형, 질병, 패널 유형, 최종 사용자라는 5개 세그먼트로 분류됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내리는 데 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
제품 및 서비스
- 시약 및 소모품
- 악기, 소프트웨어 및 액세서리
- 서비스
북미 증후군 멀티플렉스 진단 시장은 제품과 서비스를 기준으로 시약 및 소모품, 기기, 소프트웨어 및 액세서리, 서비스로 구분됩니다.
감염 유형
- 바이러스의
- 박테리아
- 기생충
- 곰팡이
감염 유형을 기준으로 북미 증후군 멀티플렉스 진단 시장은 바이러스, 박테리아, 기생충 및 진균으로 구분됩니다.
질병
- 호흡기 감염
- 위장염
- 성병 감염
- 부패
- 수막염
- 기타
북미 증후군 멀티플렉스 진단 시장은 질병을 기준으로 호흡기 감염, 위장염, 성병, 패혈증, 수막염 등으로 구분됩니다.
패널 유형
- 호흡 패널
- GI-장내 패널
- 성병 패널
- 혈액-패혈증 패널
- 뇌막염 패널
- 기타
패널 유형을 기준으로, 북미 증후군 멀티플렉스 진단 시장은 호흡기 패널, 위장관 패널, 성병 패널, 혈액 패혈증 패널, 뇌막염 패널 등으로 구분됩니다.
최종 사용자
- 병원
- 임상실험실
- 제약 및 생명공학 회사
- 연구 기관
- 기타
북미 증후군 멀티플렉스 진단 시장은 최종 사용자를 기준으로 임상 실험실, 병원, 제약 및 생명 공학 회사, 연구 기관 등으로 구분됩니다.
증후군 멀티플렉스 진단 시장 지역 분석/통찰력
위에 언급된 대로, 증후군 멀티플렉스 진단 시장을 분석하고 국가, 제품 및 서비스, 감염 유형, 질병, 패널 유형 및 최종 사용자별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
보고서에서 다루는 국가는 미국, 캐나다, 멕시코입니다.
미국의 증후군 멀티플렉스 진단 시장은 감염성 질환의 유병률 증가와 조기 및 정확한 진단에 대한 수요 증가로 인해 성장할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변경 사항을 제공합니다. 신규 판매, 교체 판매, 국가 인구 통계, 질병 역학 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 판매 채널의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
증후군 멀티플렉스 진단 시장 점유율 분석
증후군 멀티플렉스 진단 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 사우디 아라비아의 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우위가 있습니다. 제공된 위의 데이터 포인트는 증후군 멀티플렉스 진단 시장과 관련된 회사의 초점에만 관련이 있습니다.
북미 증후군 멀티플렉스 진단 시장에서 활동하는 주요 기업으로는 BioFire Diagnostics(bioMérieux SA의 자회사), Seegene Inc., Luminex Corporation. A DiaSorin Company, F. Hoffmann-La Roche Ltd, BD, Bio-Rad Laboratories, Inc., Cepheid(Danaher의 자회사), QIAGEN, Abbott, Hologic, Inc., Thermo Fisher Scientific Inc., Siemens Healthcare GmbH, Akonni Biosystems, Inc., Biocartis, QuantuMDx Group Ltd., Applied BioCode, Inc., Prominex Inc., Nanomix, Inc., Curetis(OpGen, Inc.의 자회사) 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT AND SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
4.3 MARKET SHARE PER PANEL, FOR TOP 3 PLAYERS (2021)
5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF INFECTIOUS DISEASES
6.1.2 RISING ADOPTION OF MOLECULAR DIAGNOSTICS TECHNIQUES
6.1.3 INCREASING REGULATORY APPROVAL FOR SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-COV-2) TESTING
6.1.4 INCREASING DEMAND FOR FAST AND ACCURATE DIAGNOSTIC RESULTS
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSTIC PRODUCTS
6.2.2 EXPLICIT LIMITATION OF SYNDROMIC MULTIPLEX DIAGNOSTIC
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES TAKEN BY MARKET PLAYERS
6.3.2 RISING DIAGNOSTIC HEALTHCARE EXPENDITURE
6.3.3 INTRODUCTION OF TECHNOLOGICAL ADVANCED PRODUCTS
6.4 CHALLENGES
6.4.1 PRODUCT RECALLS
6.4.2 LACK OF SKILLED PROFESSIONALS AND BARRIERS FACED IN CONDUCTING DIAGNOSTIC TESTS
7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES
7.1 OVERVIEW
7.2 REAGENTS & CONSUMABLES
7.3 INSTRUMENTS, SOFTWARE & ACCESSORIES
7.4 SERVICES
8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE
8.1 OVERVIEW
8.2 VIRAL
8.2.1 CORONAVIRUS
8.2.2 INFLUENZA VIRUS
8.2.3 ADENOVIRUS
8.2.4 RHINOVIRUS
8.2.5 ROTAVIRUS
8.2.6 OTHERS
8.3 BACTERIAL
8.3.1 PNEUMONIAE
8.3.2 BORDETELLA PERTUSSIS
8.3.3 STAPHYLOCOCCUS
8.3.4 OTHERS
8.4 PARASITES
8.5 FUNGAL
9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE
9.1 OVERVIEW
9.2 RESPIRATORY INFECTIONS
9.3 GASTROENTERITIS
9.4 SEXUALLY TRANSMITTED INFECTIONS
9.5 SEPSIS
9.6 MENINGITIS
9.7 OTHERS
10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE
10.1 OVERVIEW
10.2 RESPIRATORY PANEL
10.3 GI-ENTERIC PANEL
10.4 SEXUALLY TRANSMITTED DISEASE PANEL
10.5 BLOOD-SEPSIS PANEL
10.6 MENINGITIS PANEL
10.7 OTHERS
11 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICAL LABORATORIES
11.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
11.5 RESEARCH INSTITUTES
11.6 OTHERS
12 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 BIOFIRE DIAGNOSTICS (A SUBSIDIARY OF BIOMÉRIEUX SA)
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION. A DIASORIN COMPANY
15.3.1 COMPANY SNAPSHOT
15.3.2 RECENT FINANCIALS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 CEPHEID (A SUBSIDIARY OF DANAHER)
15.4.1 COMPANY SNAPSHOT
15.4.2 RECENT FINANCIALS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 QIAGEN
15.5.1 COMPANY SNAPSHOT
15.5.2 RECENT FINANCIALS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AKONNI BIOSYSTEMS, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 APPLIED BIOCODE, INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 BD
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOCARTIS
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 BIO-RAD LABORATORIES, INC.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 BOSCH HEALTHCARE SOLUTIONS GMBH (A SUBSIDIARY OF ROBERT BOSCH GMBH)
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 CURETIS (A SUBSIDIARY OF OPGEN, INC.)
15.13.1 COMPANY SNAPSHOT
15.13.2 RECENT FINANCIALS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HOLOGIC, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 RECENT FINANCIALS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 MIRXES PTE LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NANŌMIX, INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 PROMINEX INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 QUANTUMDX GROUP LTD.
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 SEEGENE INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 SIEMENS HEALTHCARE GMBH
15.20.1 COMPANY SNAPSHOT
15.20.2 RECENT FINANCIALS
15.20.3 PRODUCT PORTFOLIO
15.20.4 RECENT DEVELOPMENT
15.21 THERMOFISHER SCIENTIFIC INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 RECENT FINANCIALS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
표 목록
TABLE 1 COST OF THE PRODUCT
TABLE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA REAGENTS & CONSUMABLES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA INSTRUMENTS, SOFTWARE & ACCESSORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA SERVICES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA PARASITES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA FUNGAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA RESPIRATORY INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA GASTROENTERITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA SEXUALLY TRANSMITTED INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA SEPSIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA MENINGITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA RESPIRATORY PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA GI-ENTERIC PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA SEXUALLY TRANSMITTED DISEASE PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA BLOOD-SEPSIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA MENINGITIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA HOSPITALS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA CLINICAL LABORATORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA RESEARCH INSTITUTES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 42 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 43 U.S. VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 44 U.S. BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 45 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 46 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 47 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 49 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 50 CANADA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 51 CANADA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 52 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 53 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 54 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 56 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 57 MEXICO VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 58 MEXICO BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 59 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 60 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 61 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE AND INCIDENCE OF INFECTIOUS DISEASES IS EXPECTED TO DRIVE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 REAGENTS & CONSUMABLES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
FIGURE 15 TOTAL CASES OF COVID-19 IN NORTH AMERICA
FIGURE 16 TOTAL CASES OF COVID-19 IN EUROPE
FIGURE 17 NATIONAL HEALTH EXPENDITURE VS MEDICAL DEVICE EXPENDITURE, U.S., 2019
FIGURE 18 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2021
FIGURE 19 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2022-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2021
FIGURE 23 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2022-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2021
FIGURE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2022-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2021
FIGURE 31 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2022-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 41 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 42 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT AND SERVICES (2022-2029)
FIGURE 43 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.