>북미 비강 스프레이 시장, 제품 유형별(비강 충혈 완화 스프레이, 스테로이드 비강 스프레이, 식염수 용액/식염수 비강 스프레이 및 기타), 용기 디자인(펌프 병 및 가압 캐니스터), 투여 형태(다중 투여, 단위/단일 투여 및 2회 투여), 치료적 분류(항히스타민제, 비강 스테로이드, 비만 세포 억제제 및 항콜린제), 응용 분야(비강 충혈, 알레르기 및 비알레르기 비염, 중추 신경계 장애, 예방 접종 및 기타), 처방/이용 가능성(처방전 없이 구입 가능한 약 및 처방약), 최종 사용자(가정 요양 시설, 병원, 진료소 및 지역 사회 건강 관리) - 업계 동향 및 2030년까지의 예측.
북미 비강 스프레이 시장 분석 및 통찰력
알레르기성 비염 은 전 세계 5억 명 이상의 사람들에게 문제를 일으키는 주요 건강 문제입니다. 비강 막힘, 코골이, 후비루, 기침, 후각 감소, 두통 등의 증상이 지구상 환자의 거의 절반에게 수개월 또는 수년간 나타납니다. 이러한 증상은 환자의 삶의 질에도 영향을 미칠 수 있습니다.
비강 스프레이는 비강 막힘, 감염, 가려움증이나 눈물, 콧물, 가슴 막힘, 기침, 천명, 호흡 곤란, 얕은 호흡 등과 같은 주요 알레르기 문제를 치료하기 위한 비강 약물 전달 시스템으로 널리 사용되는 장치입니다. 비강 용종, 염증성 장 질환, 신장 관련 문제 등을 포함하는 다른 질병도 있습니다. 스프레이의 도움으로 복용 형태로 코로 약물이나 약물을 투여하는 것은 환자의 신체에서 약물의 빠른 작용을 제공하는 비침습적 기술입니다. 비강 스프레이는 가장 비용 효율적인 방법 중 하나이며 침습이 필요하지 않으며 사용하기 쉽고 스스로 투여할 수 있으며 환자의 준수도가 높습니다. 따라서 비강 약물 전달은 최근 인기 있는 약물 투여 경로 중 하나이며 강력한 성장 기회가 있습니다.
비강 스프레이는 비강 알레르기, 코막힘, 만성 부비동염, 알레르기성 비염 및 코와 관련된 기타 문제와 같은 비강 질환을 치료하기 위한 일반 의약품(OTC) 또는 처방약으로 시중에 판매됩니다. 이러한 스프레이는 펌프 또는 캐니스터 형태로 시중에 판매됩니다.
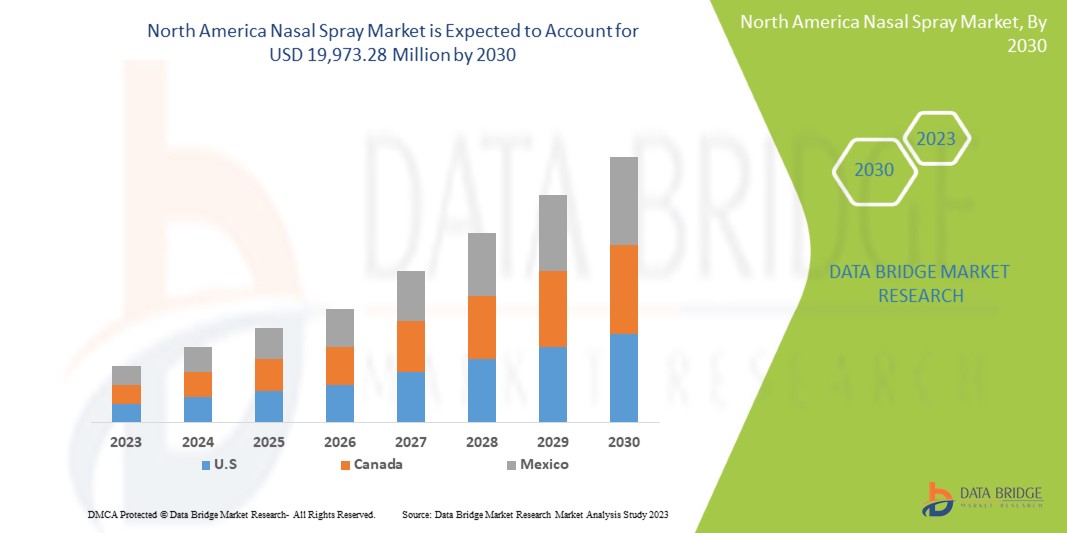
Data Bridge Market Research에 따르면, 북미 비강 스프레이 시장은 2030년까지 199억 7,328만 달러 규모에 도달할 것으로 예상되며, 이는 예측 기간 동안 연평균 성장률 6.8%를 기록할 것으로 예상됩니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2023년부터 2030년까지 |
|
기준 연도 |
2022 |
|
역사적 해 |
2021 (2015-2020까지 사용자 정의 가능) |
|
양적 단위 |
매출은 백만, 볼륨은 단위, 가격은 USD로 표시 |
|
다루는 세그먼트 |
Product Type (Decongestion Nasal Spray, Steroid Nasal Spray, Salt Water Solution/Saline Nasal Spray, and Others), Container Design (Pump Bottles and Pressurized Canisters), Dosage Form (Multi Dose, Unit/Single Dose, and Bi Dose), Therapeutic Class (Antihistamine, Nasal Steroid, Mast Cell Inhibitor, and Anticholinergic), Application (Nasal Congestion, Allergic and Non-Allergic Rhinitis, Central Nervous System Disorders, Vaccination, and Others), Prescription/Availability (Over The Counter and Prescribed), End User (Home Care Settings, Hospitals, Clinics, and Community Health Care) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
EMERGENT, Cipla Inc., Sandoz International GmbH (A Part of Novartis), Aytu Health (A subsidiary of Aytu BioPharma, Inc.), Bayer AG, GlaxoSmithKline plc., Assertio Therapeutics, Inc., Aurena Laboratories., J Pharmaceuticals, St. Renatus., Ultratech India Limited, Catalent, Inc., Teva Pharmaceuticals USA, Inc. (A subsidiary of Teva Pharmaceutical Industries Ltd), Pfizer Inc., Viatris Inc., LEEFORD HEALTHCARE LTD, and Aishwarya Group among others |
Market Definition
Nasal sprays are devices that help in the delivery of the drug through the nose in the nasal cavity to treat allergies and other problems related to brain inflammation, diabetes, dental problems, and the central nervous system. Allergy is a condition in which the immune system of the body reacts abnormally to a foreign substance. Nasal spray drug products consist of therapeutically active ingredients or drug substances in the form of solution or suspension of excipients.
There are different types of nasal spray available in the market, such as decongestion nasal spray, saline nasal spray, and steroid nasal spray. Decongestants are devices with drugs for the treatment of nasal congestion and provide short-term relief from a blocked nose. The decongestion nasal spray consists of drugs that open up the airways of the nasal part and helps in the breathing process.
During the cold and dry winter season, saline nasal sprays are used to avoid problems related to a stuffy nose. These devices are used to treat sinusitis, nasal crusting, and thin secretions of liquid from the nose, which are formed due to bacterial infection and lead to the dryness of the nasal area. This device helps in cleaning the nasal passage and mucus present in that area due to cold.
North America Nasal Spray Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
DRIVER
- Increase in Infection and Allergic Cases
Allergy is a condition that makes the immune system weaken, and the immune system reacts abnormally to a foreign substance. Allergies have many forms, such as Allergic Rhinitis (AR), and this affects nearly 40 to 50 million people in the U.S. Some allergies may interfere with day-to-day activities and the quality of life.
Due to the increase in population, urbanization, and industrialization has increased tremendously, which has led to a rise in allergen. This allergen is responsible for allergies or infectious diseases in many people.
Thus, the rise in the prevalence of allergic diseases such as AR has led to an increase in the demand for nasal sprays, which is expected to drive market growth in the coming years.
RESTRAINT
- Adverse Effects of Using Nasal Sprays
Many people opt for nasal sprays in order to get relief from a runny and stuffy nose, but using a nasal spray for a longer period of time causes several side effects. Steroid nasal sprays, also called corticosteroid nasal sprays, are anti-inflammatory medicines that are sprayed into the nose. They can be used to treat a range of conditions, including hay fever, sinusitis, non-allergic rhinitis, and nasal polyps. Some steroid nasal sprays are available to buy from pharmacies and shops, while others are only available on prescription.
Using nasal sprays for a prolonged period of time can lead to bleeding from the nose and even headaches. Some may experience other side effects, such as addiction or even congestion.
If one is taking a high dose for a long time, there is also a small chance one could get some of the side effects of steroid tablets, such as increased appetite, mood changes, and difficulty sleeping.
Thus, the adverse effects of nasal sprays are expected to restrain the market growth.
OPPORTUNITY
-
Expanding Therapeutic Applications for Nasal Sprays
Intranasal administration provides various useful options for the local and systemic delivery of diverse therapeutic agents for the treatment of problems such as allergies, respiratory diseases, and many others.
A nasal spray is one of the recently developed products, such as OptiNose and ViaNase, that helps to enable the targeting of formulations to a specific area of the site in the nasal cavity in humans.
Nasal therapy has been known as a form of treatment in Indian medicine for decades. It is also called “NASAYA KARMA” in Ayurvedic systems in Indian medicine.
The nasal spray consists of one or more than one therapeutically active ingredients in the form of suspension or solution in a non-pressurized dispenser. The most common allergy is AR.
Apart from respiratory problems, there are other therapeutic areas such as orthopedic, renal, and many other areas, where the nasal spray is playing important role in treating and curing such problems. In the future, other treatments may be developed that can help in the treatment of other problems easily.
CHALLENGE
- Regulatory Hurdles
Nasal drug delivery can be evaluated by many regulatory agencies, such as the U.S. FDA and the European Medicines Agency (EMA). These agencies provide a set of guidelines and regulations for any product before it is launched in the market. They allow performing various in vitro test methods for determining the characterization of nasal drug products and that should come in an acceptable range as given by such regulatory bodies.
Nasal spray can be designed as per the dose requirement of drug substances for patients suffering from nasal or any other issues. Some aspects of nasal sprays may be exceptional in the case of formulation, manufacturing, container closure system, stability, controls of critical steps, intermediates, and drug products. These aspects should be measured carefully while developing a program of nasal spray. Because of these changes ability of the product to treat patients may get affected.
Strict rules and regulations for the product for nasal spray manufacturing are more challenging for the manufacturer to get approval from regulatory agencies. The spray characteristics can be influenced by the design of the device, and by the handling of the device. Performing such tests to get the perfect result, that is, an acceptable range as given by the regulatory body, is more challenging in nasal spray manufacturing.
Post-COVID-19 Impact Analysis on the North America Nasal Spray Market
COVID-19 had a significant impact on the generic drug market for inhalation and nasal spray, as one of the main symptoms associated with COVID-19 is shortness of breath. The demand for inhalation and nasal spray increased during the pandemic.
For instance,
- According to a MedComm journal article published in 2021, nasal sprays demonstrated the ability to be an effective COVID-19 treatment and vaccine option
Spray formulations that may inactivate SARS-CoV-2 or restrict its entry into cells were thought to be beneficial in preventing viral spread to the lungs or surrounding people. Relaxed lockdowns and the resumption of healthcare practices are expected to accelerate market growth in the post-pandemic era. COVID-19 patients are still experiencing long-term side effects such as shortness of breath.
Recent Developments
- In January 2023, Viatris Inc. (NASDAQ: VTRS), a North America healthcare company, today announced that it has closed its acquisitions of Oyster Point Pharma and Famy Life Sciences to establish a new Viatris Eye Care Division. This acquisition would help the company in its business expansion.
- In March 2023, Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd, announced the launch of additional strengths for the generic version of Revlimid1 (lenalidomide capsules), in 2.5 mg, and 20 mg strengths, in the U.S. This product launch would help the company in its product portfolio expansion.
North America Nasal Spray Market Scope
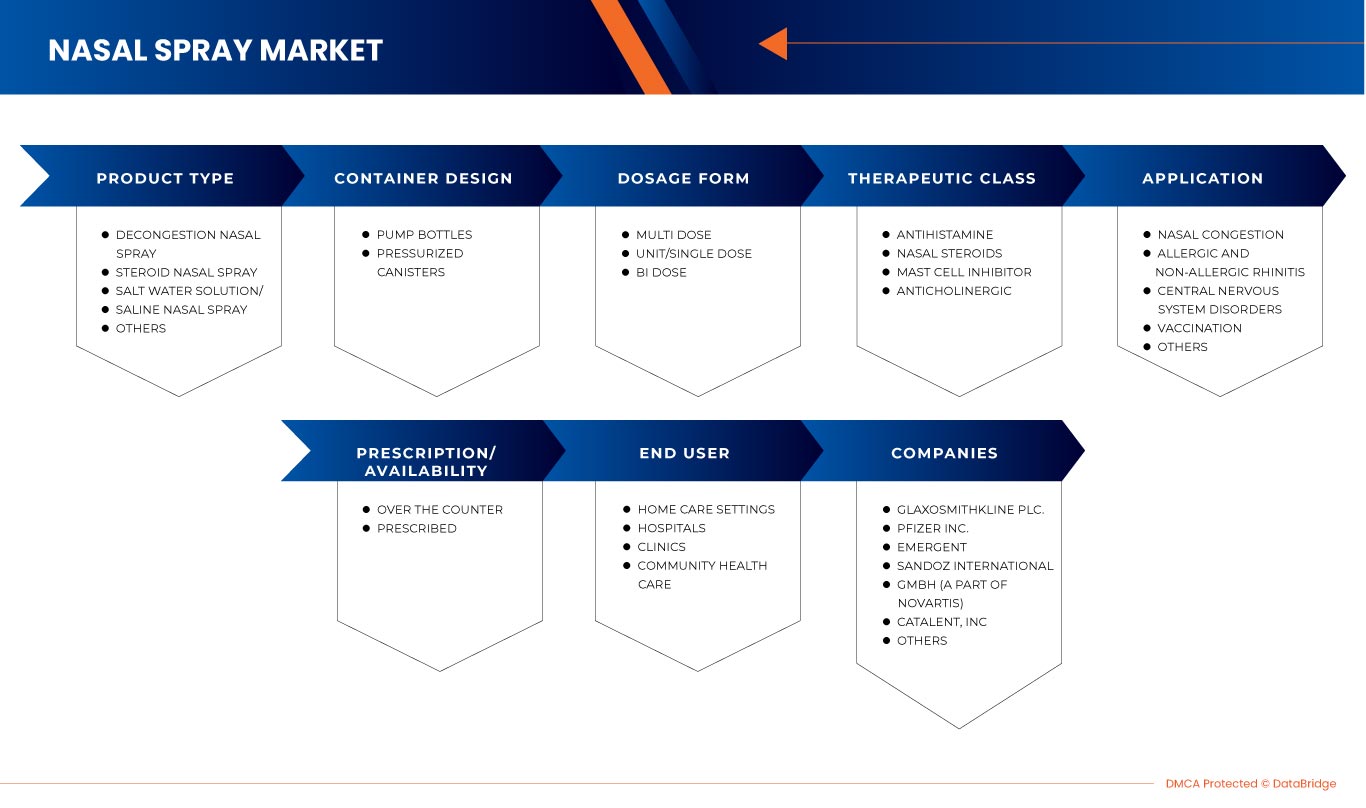
The North America nasal spray market is segmented into seven notable segments on the basis of product type, container design, dosage form, therapeutic class, application, prescription/ availability, and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
PRODUCT TYPE
- DECONGESTION NASAL SPRAY
- STEROID NASAL SPRAY
- SALT WATER SOLUTION/ SALINE NASAL SPRAY
- OTHERS
On the basis of product type, the market is segmented into decongestion nasal spray, salt water solution/saline nasal spray, steroid nasal spray, and others.
CONTAINER DESIGN
- PUMP BOTTLES
- PRESSURIZED CANISTERS
On the basis of container design, the market is segmented into pressurized canisters and pump bottles.
DOSAGE FORM
- MULTI DOSE
- UNIT/SINGLE DOSE
- BI DOSE
On the basis of dosage form, the market is segmented into unit/single dose, bi dose, and multi dose.
THERAPEUTIC CLASS
- ANTIHISTAMINE
- NASAL STEROIDS
- MAST CELL INHIBITOR
- ANTICHOLINERGIC
On the basis of therapeutic class, the market is segmented into antihistamine, nasal steroids, mast cell inhibitor, and anticholinergic.
APPLICATION
- NASAL CONGESTION
- ALLERGIC AND NON-ALLERGIC RHINITIS
- CENTRAL NERVOUS SYSTEM DISORDERS
- VACCINATION
- OTHERS
On the basis of application, the market is segmented into nasal congestion, allergic and non-allergic rhinitis, central nervous system disorders, vaccination, and others.
PRESCRIPTION/AVAILABILITY
- OVER THE COUNTER
- PRESCRIBED
On the basis of prescription/availability, the market is segmented into over the counter and prescribed.
END USER
- HOME CARE SETTINGS
- HOSPITALS
- CLINICS
- COMMUNITY HEALTH CARE
On the basis of end user, the market is segmented into home care settings, hospitals, clinics, and community health care.
North America Nasal Spray Market Regional Analysis/Insights
The North America nasal spray market is segmented into seven notable segments on the basis of product type, container design, dosage form, therapeutic class, application, prescription/ availability, and end user.
The countries covered in this market report are U.S., Canada, and Mexico.
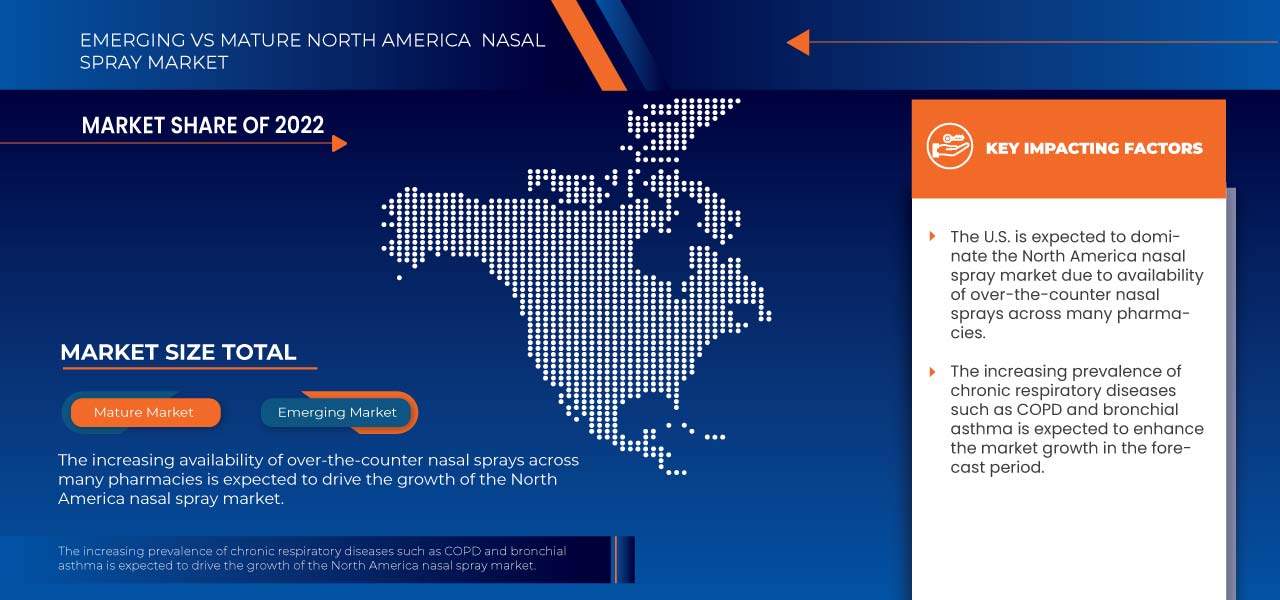
In 2023, the U.S. is dominating the North America nasal spray market due to the rise in allergic diseases, which is driving the market growth.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Nasal Spray Market Share Analysis
북미 비강 스프레이 시장 경쟁 구도는 경쟁자에 대한 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, R&D 투자, 새로운 시장 이니셔티브, 생산 현장 및 시설, 회사의 강점과 약점, 제품 출시, 제품 승인, 제품 폭과 범위, 적용 우세, 제품 유형 수명선 곡선입니다. 제공된 위의 데이터 포인트는 회사가 시장에 집중하는 것과만 관련이 있습니다.
북미 비강 스프레이 시장에서 활동하는 주요 기업으로는 EMERGENT, Cipla Inc., Sandoz International GmbH(Novartis의 일부), Aytu Health(Aytu BioPharma, Inc.의 자회사), Bayer AG, GlaxoSmithKline plc., Assertio Therapeutics, Inc., Aurena Laboratories., J Pharmaceuticals, St. Renatus., Ultratech India Limited, Catalent, Inc., Teva Pharmaceuticals USA, Inc.(Teva Pharmaceutical Industries Ltd의 자회사), Pfizer Inc., Viatris Inc., LEEFORD HEALTHCARE LTD, Aishwarya Group 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES MODEL
4.2 PESTEL ANALYSIS
5 INDUSTRY INSIGHTS: NORTH AMERICA NASAL SPRAY MARKET
5.1 INDUSTRY INSIGHTS
6 REGULATIONS: NORTH AMERICA NASAL SPRAY MARKET
6.1 REGULATORY SCENARIO IN THE U.S.
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 INCREASE IN INFECTION AND ALLERGIC CASES
7.1.2 RISING PREVALENCE OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) AND ASTHMA
7.1.3 EFFECTIVENESS OVER OTHER FORMS OF DRUG DELIVERY
7.1.4 IMPROVING PATIENT COMPLIANCE AND ACCEPTABILITY
7.2 RESTRAINTS
7.2.1 PRODUCT RECALLS
7.2.2 ADVERSE EFFECTS OF USING NASAL SPRAYS
7.3 OPPORTUNITIES
7.3.1 NEW RISING DEMAND FOR SELF-ADMINISTRATIVE DRUG DELIVERY
7.3.2 EXPANDING THERAPEUTIC APPLICATIONS FOR NASAL SPRAYS
7.3.3 STRATEGIC INITIATIVES TAKEN BY KEY MARKET PLAYERS
7.4 CHALLENGES
7.4.1 REGULATORY HURDLES
7.4.2 ADDICTION TO NASAL SPRAY
8 NORTH AMERICA NASAL SPRAY MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 DECONGESTION NASAL SPRAY
8.3 STEROID NASAL SPRAY
8.4 SALT WATER SOLUTION/SALINE NASAL SPRAY
8.5 OTHERS
9 NORTH AMERICA NASAL SPRAY MARKET, BY CONTAINER DESIGN
9.1 OVERVIEW
9.2 PUMP BOTTLES
9.3 PRESSURIZED CANISTERS
10 NORTH AMERICA NASAL SPRAY MARKET, BY DOSAGE FORM
10.1 OVERVIEW
10.2 MULTI DOSE
10.3 UNIT/SINGLE DOSE
10.4 BI DOSE
11 NORTH AMERICA NASAL SPRAY MARKET, BY THERAPEUTIC CLASS
11.1 OVERVIEW
11.2 ANTIHISTAMINE
11.3 NASAL STEROIDS
11.4 MAST CELL INHIBITOR
11.5 ANTICHOLINERGIC
12 NORTH AMERICA NASAL SPRAY MARKET, BY APPLICATION
12.1 OVERVIEW
12.2 NASAL CONGESTION
12.3 ALLERGIC AND NON-ALLERGIC RHINITIS
12.4 CENTRAL NERVOUS SYSTEM DISORDERS
12.5 VACCINATION
12.6 OTHERS
13 NORTH AMERICA NASAL SPRAY MARKET, BY PRESCRIPTION/AVAILABILITY
13.1 OVERVIEW
13.2 PRESCRIBED
13.3 OVER THE COUNTER
14 NORTH AMERICA NASAL SPRAY MARKET, BY END USER
14.1 OVERVIEW
14.2 HOME CARE SETTINGS
14.3 HOSPITALS
14.4 CLINICS
14.5 COMMUNITY HEALTH CARE
15 NORTH AMERICA NASAL SPRAY MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA NASAL SPRAY MARKET, COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 GLAXOSMITHKLINE PLC.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 PFIZER INC.
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 EMERGENT
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 SANDOZ INTERNATIONAL GMBH (A PART OF NOVARTIS)
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENT
18.5 CATALENT, INC.
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
18.6 AYTU HEALTH (A SUBSIDIARY OF AYTU BIOPHARMA, INC.)
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 AISHWARYA GROUP
18.7.1 COMPANY SNAPSHOT
18.7.2 PRODUCT PORTFOLIO
18.7.3 RECENT DEVELOPMENTS
18.8 ASSERTIO THERAPEUTICS, INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 REVENUE ANALYSIS
18.8.3 PRODUCT PORTFOLIO
18.8.4 RECENT DEVELOPMENT
18.9 AURENA LABORATORIES.
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENTS
18.1 BAYER AG
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 COMPANY SHARE ANALYSIS
18.10.4 PRODUCT PORTFOLIO
18.10.5 RECENT DEVELOPMENTS
18.11 CIPLA INC.
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENT
18.12 J PHARMACEUTICALS.
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 LEEFORD HEALTHCARE LTD
18.13.1 COMPANY SNAPSHOT
18.13.2 PRODUCT PORTFOLIO
18.13.3 RECENT DEVELOPMENTS
18.14 ST. RENATUS.
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENTS
18.15 TEVA PHARMACEUTICALS USA, INC. (A SUBSIDIARY OF TEVA PHARMACEUTICAL INDUSTRIES LTD)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENTS
18.16 ULTRATECH INDIA LIMITED
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENTS
18.17 VIATRIS INC.
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 COMPANY SHARE ANALYSIS
18.17.4 PRODUCT PORTFOLIO
18.17.5 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
표 목록
TABLE 1 NORTH AMERICA NASAL SPRAY MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA DECONGESTION NASAL SPRAY IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA STEROID NASAL SPRAY IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA SALT WATER SOLUTION/SALINE NASAL SPRAY IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA OTHERS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA NASAL SPRAY MARKET, BY CONTAINER DESIGN, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA PUMP BOTTLES IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA PRESSURIZED CANISTERS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA NASAL SPRAY MARKET, BY DOSAGE FORM, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA MULTI DOSE IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA UNIT/SINGLE DOSE IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA BI DOSE IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NASAL SPRAY MARKET, BY THERAPEUTIC CLASS, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA ANTIHISTAMINE IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA NASAL STEROIDS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA MAST CELL INHIBITOR IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA ANTICHOLINERGIC IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA NASAL SPRAY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA NASAL CONGESTION IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA ALLERGIC AND NON-ALLERGIC RHINITIS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA CENTRAL NERVOUS SYSTEM DISORDERS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA VACCINATION IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA OTHERS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA NASAL SPRAY MARKET, BY PRESCRIPTION/AVAILABILITY, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA PRESCRIBED IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA OVER THE COUNTER IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA NASAL SPRAY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA HOME CARE SETTINGS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA HOSPITALS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA CLINICS IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA COMMUNITY HEALTH CARE IN NASAL SPRAY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA NASAL SPRAY MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA NASAL SPRAY MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA NASAL SPRAY MARKET, BY CONTAINER DESIGN, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA NASAL SPRAY MARKET, BY DOSAGE FORM, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA NASAL SPRAY MARKET, BY THERAPEUTIC CLASS, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA NASAL SPRAY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA NASAL SPRAY MARKET, BY PRESCRIPTION/AVAILABILITY, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA NASAL SPRAY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 40 U.S. NASAL SPRAY MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 41 U.S. NASAL SPRAY MARKET, BY CONTAINER DESIGN, 2021-2030 (USD MILLION)
TABLE 42 U.S. NASAL SPRAY MARKET, BY DOSAGE FORM, 2021-2030 (USD MILLION)
TABLE 43 U.S. NASAL SPRAY MARKET, BY THERAPEUTIC CLASS, 2021-2030 (USD MILLION)
TABLE 44 U.S. NASAL SPRAY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 45 U.S. NASAL SPRAY MARKET, BY PRESCRIPTION/AVAILABILITY, 2021-2030 (USD MILLION)
TABLE 46 U.S. NASAL SPRAY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 CANADA NASAL SPRAY MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 CANADA NASAL SPRAY MARKET, BY CONTAINER DESIGN, 2021-2030 (USD MILLION)
TABLE 49 CANADA NASAL SPRAY MARKET, BY DOSAGE FORM, 2021-2030 (USD MILLION)
TABLE 50 CANADA NASAL SPRAY MARKET, BY THERAPEUTIC CLASS, 2021-2030 (USD MILLION)
TABLE 51 CANADA NASAL SPRAY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 52 CANADA NASAL SPRAY MARKET, BY PRESCRIPTION/AVAILABILITY, 2021-2030 (USD MILLION)
TABLE 53 CANADA NASAL SPRAY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 54 MEXICO NASAL SPRAY MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 MEXICO NASAL SPRAY MARKET, BY CONTAINER DESIGN, 2021-2030 (USD MILLION)
TABLE 56 MEXICO NASAL SPRAY MARKET, BY DOSAGE FORM, 2021-2030 (USD MILLION)
TABLE 57 MEXICO NASAL SPRAY MARKET, BY THERAPEUTIC CLASS, 2021-2030 (USD MILLION)
TABLE 58 MEXICO NASAL SPRAY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 59 MEXICO NASAL SPRAY MARKET, BY PRESCRIPTION/AVAILABILITY, 2021-2030 (USD MILLION)
TABLE 60 MEXICO NASAL SPRAY MARKET, BY END USER, 2021-2030 (USD MILLION)
그림 목록
FIGURE 1 NORTH AMERICA NASAL SPRAY MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA NASAL SPRAY MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA NASAL SPRAY MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA NASAL SPRAY MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA NASAL SPRAY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA NASAL SPRAY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA NASAL SPRAY MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA NASAL SPRAY MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA NASAL SPRAY MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA NASAL SPRAY MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN INFECTION AND ALLERGIC CASES IS EXPECTED TO DRIVE THE NORTH AMERICA NASAL SPRAY MARKET IN THE FORECAST PERIOD
FIGURE 12 THE DECONGESTION NASAL SPRAY SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA NASAL SPRAY MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA NASAL SPRAY MARKET
FIGURE 14 NORTH AMERICA NASAL SPRAY MARKET: BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA NASAL SPRAY MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA NASAL SPRAY MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA NASAL SPRAY MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA NASAL SPRAY MARKET: BY CONTAINER DESIGN, 2022
FIGURE 19 NORTH AMERICA NASAL SPRAY MARKET: BY CONTAINER DESIGN, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA NASAL SPRAY MARKET: BY CONTAINER DESIGN, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA NASAL SPRAY MARKET: BY CONTAINER DESIGN, LIFELINE CURVE
FIGURE 22 NORTH AMERICA NASAL SPRAY MARKET: BY DOSAGE FORM, 2022
FIGURE 23 NORTH AMERICA NASAL SPRAY MARKET: BY DOSAGE FORM, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA NASAL SPRAY MARKET: BY DOSAGE FORM, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA NASAL SPRAY MARKET: BY DOSAGE FORM, LIFELINE CURVE
FIGURE 26 NORTH AMERICA NASAL SPRAY MARKET: BY THERAPEUTIC CLASS, 2022
FIGURE 27 NORTH AMERICA NASAL SPRAY MARKET: BY THERAPEUTIC CLASS, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA NASAL SPRAY MARKET: BY THERAPEUTIC CLASS, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA NASAL SPRAY MARKET: BY THERAPEUTIC CLASS, LIFELINE CURVE
FIGURE 30 NORTH AMERICA NASAL SPRAY MARKET: BY APPLICATION, 2022
FIGURE 31 NORTH AMERICA NASAL SPRAY MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA NASAL SPRAY MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA NASAL SPRAY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 34 NORTH AMERICA NASAL SPRAY MARKET: BY PRESCRIPTION/AVAILABILITY, 2022
FIGURE 35 NORTH AMERICA NASAL SPRAY MARKET: BY PRESCRIPTION/AVAILABILITY, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA NASAL SPRAY MARKET: BY PRESCRIPTION/AVAILABILITY, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA NASAL SPRAY MARKET: BY PRESCRIPTION/AVAILABILITY, LIFELINE CURVE
FIGURE 38 NORTH AMERICA NASAL SPRAY MARKET: BY END USER, 2022
FIGURE 39 NORTH AMERICA NASAL SPRAY MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA NASAL SPRAY MARKET: BY END USER, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA NASAL SPRAY MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 NORTH AMERICA NASAL SPRAY MARKET: SNAPSHOT (2022)
FIGURE 43 NORTH AMERICA NASAL SPRAY MARKET: BY COUNTRY (2022)
FIGURE 44 NORTH AMERICA NASAL SPRAY MARKET: BY COUNTRY (2023 & 2030)
FIGURE 45 NORTH AMERICA NASAL SPRAY MARKET: BY COUNTRY (2022 & 2030)
FIGURE 46 NORTH AMERICA NASAL SPRAY MARKET: PRODUCT TYPE (2023-2030)
FIGURE 47 NORTH AMERICA NASAL SPRAY MARKET: COMPANY SHARE 2022 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.