North America CRISPR Gene Detection and Diagnostic Market, By Class (Class 1- Multiple Effector Proteins and Class 2 -Single CrRNA-Binding Protein), Products & Services (Products and Services), Application (Biomedical Diagnostics, Genome Engineering, Drug Discovery, Agricultural Applications and Others), Workflow (Sample Preparation, Pre-Amplification, CrRNA, Cas Enzymes and Sensing), End User (Hospitals, Diagnostic Centers, Biotechnology Companies, Academic and Research Institutes and Others), Distribution Channel (Direct Tender, Retail Sales) Industry Trends and Forecast to 2029
Market Definition and Insights
CRISPR is clustered regularly interspaced short palindromic repeats and is a tool for genome editing, it allows researchers to alter DNA sequences and modify gene function easily. It has many potential applications, including correcting genetic defects and treating and preventing the spread of diseases. CRISPR-based diagnostics have been used for many biomedical applications, such as sensing nucleic-acid-based biomarkers of infectious and non-infectious diseases and detecting genetic diseases. The assay kits in CRISPR are composed of two components: a protein called Cas9 and a guide RNA, a string of nucleic acid molecules with a certain genetic code.
This CRISPR-Cas9 system has been modified for use in mammalian cells. We can either knock out specific genes by introducing a guide sequence (sgRNA) specific to our gene of interest by introducing frameshift mutations via Non-Homologous End Joining (NHEJ) or generate knock-in mutations.
CRISPR-Cas 9 systems have extended the scope of diagnostics and services in gene and cell therapies. Pharmaceutical companies invest heavily in R&D to develop new products, with a surge of gene and cell therapy agents entering early development. The market players investing would allow producing safe and effective treatments for patients in serious need.
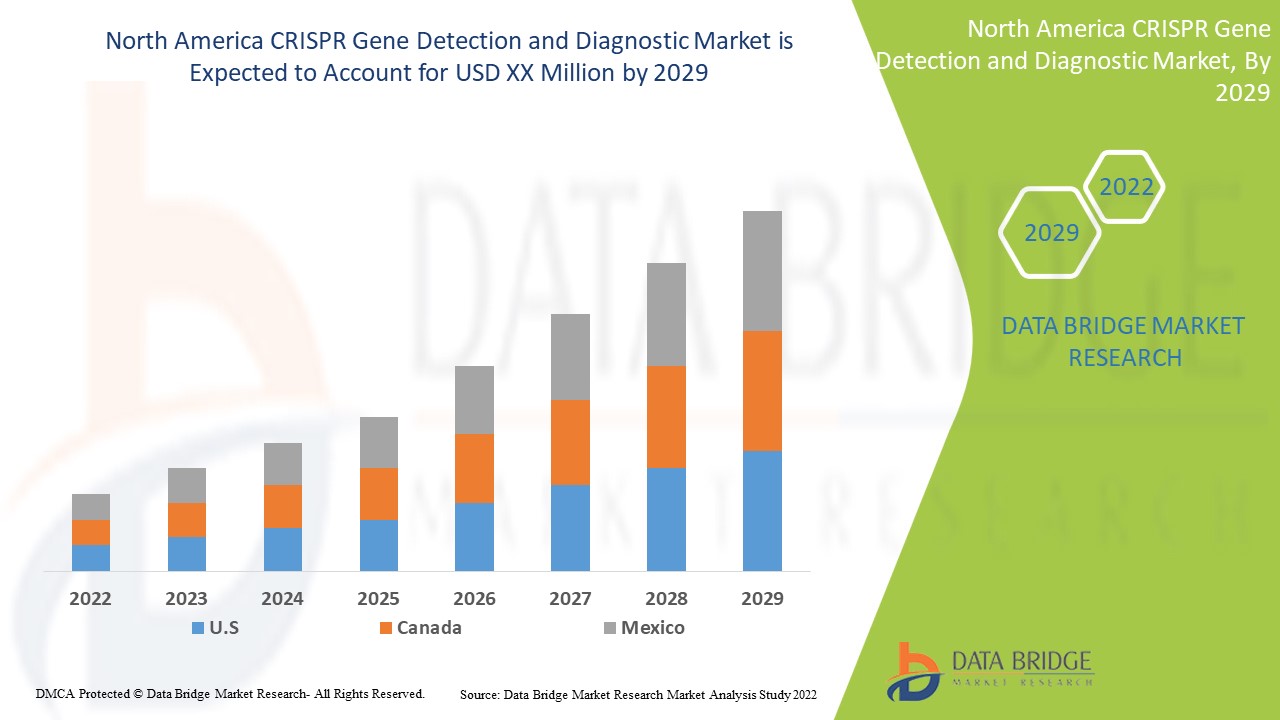
The North America CRISPR gene detection and diagnostic is supportive and aims to reduce the severity of the symptoms. Data Bridge Market Research analyses that the CRISPR gene detection and diagnostic market will grow at a CAGR of 19.6 % during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
분류별(분류 1- 다중 효과 단백질 및 분류 2- 단일 CrRNA 결합 단백질), 제품 및 서비스(제품 및 서비스), 응용 분야(생물의학 진단, 게놈 엔지니어링, 약물 발견, 농업 응용 분야 및 기타), 워크플로(샘플 준비, 사전 증폭, CrRNA, Cas 효소 및 감지), 최종 사용자(병원, 진단 센터, 생명 공학 회사, 학술 및 연구 기관 및 기타) 유통 채널(직접 입찰, 소매 판매) |
|
적용 국가 |
미국, 캐나다, 멕시코 |
|
시장 참여자 포함 |
GenScript, OriGene Technologies, Inc., Applied StemCell, GeneCopoeia, Inc., Agilent Technologies, Inc., Synthego, BioVision Inc., Hera Biolabs, Cellecta, Inc., New England Biolabs, 10x Genomics, addgene CasTag Biosciences, Merck KGaA, Integrated DNA Technologies, Inc. (Danaher의 자회사), Thermo Fisher Scientific Inc. 등 |
북미 CRISPR 유전자 검출 및 진단 시장 동향
운전자
- 만성질환의 유병률 및 발생률 증가
만성 질환은 흔한 건강 상태로, 성인 3명 중 1명이 만성 질환을 앓고 있습니다. 만성 질환은 많은 시민의 건강과 삶의 질에 영향을 미쳤습니다.
CRISPR는 clustered regular interspaced short palindromic repeats로 축약됩니다. 최근 몇 년 동안 CRISPR는 유전자 편집을 위한 중요한 도구가 되었으며, 이는 세포의 특정 DNA 시퀀스를 변경하는 데 사용됩니다. CRISPR는 헌팅턴병, 근위축증, 암 및 고콜레스테롤의 연구 및 치료에 중요한 용도가 있습니다.
예를 들어,
- 2021년, NORD - National Organization for Rare Disorders, Inc.의 데이터는 듀센 근이영양증(DMD)의 진단된 발생률을 나타냈습니다. 듀센 근이영양증(DMD)은 전 세계적으로 3,500명의 남아 출산 중 1명에게 영향을 미치는 흔한 유전적 질환입니다.
- 연구개발 투자 증가
CRISPR-Cas 9 시스템과 같은 유전자 편집 기술은 유전자 및 세포 치료의 진단 및 서비스 범위를 확대했습니다. 제약 회사는 새로운 제품을 개발하기 위해 R&D에 많은 투자를 하고 있으며, 초기 개발에 돌입하는 유전자 및 세포 치료제가 급증하고 있습니다. 투자하는 시장 참여자는 심각한 도움이 필요한 환자를 위한 안전하고 효과적인 치료법을 생산한다는 목표를 달성할 수 있을 것입니다.
예를 들어,
- 2022년 2월, 신테고는 초기 단계 연구에서 임상 단계까지 CRISPR 기반 의약품 개발을 촉진하기 위한 연구 개발 투자로 2억 달러를 모금했습니다. 신테고는 시리즈 E 자금에서 투자한 금액을 사용하여 CRISPR 진단 및 서비스 생성을 가속화할 것입니다.
CRISPR 유전자 진단을 위한 자금 지원 가능성
CRISPR 유전자 진단 및 연구는 국립보건원(NIH) 예산으로 지원됩니다. 민간 부문도 CRISPR 유전자 검출 및 연구에 자금을 지원하지만, 이러한 투자는 일반적으로 나중에, 테스트 및 개발 단계에서, 그 다음 초기 기초 연구에서 이루어집니다. 게놈 편집이 매우 새로운 분야이기 때문에 편견 없는 정부 기관이 감독해야 합니다. FDA는 신중하고 철저하지만, 그들은 끊임없이 자금 조달에 어려움을 겪고 있으며, 지불을 잠재적 미래 수혜자와 일치시키는 장기 투자를 하고 있습니다. CRISPR 유전자 검출 및 진단 시장의 성장을 더욱 강화할 것입니다.
또한 CRISPR 유전자 진단의 발전, 공공 및 민간 기관의 인식 확산을 위한 이니셔티브 증가, 정부 자금 지원 증가는 CRISPR 유전자 검출 시장을 확대할 요인입니다. 효과적인 치료법에 대한 수요 증가, 시기적절한 진단에 대한 인식 증가와 같은 다른 요인은 CRISPR 유전자 검출 및 진단 시장의 성장률에 긍정적인 영향을 미칠 것입니다. 또한 높은 가처분 소득, 만성 질환 수 증가, 라이프스타일의 변화로 인해 CRISPR 유전자 검출 및 진단 시장이 확대될 것입니다.
기회
- 의료비 지출 증가
게다가 정부와 민간 기관의 연구 개발 활동이 늘어나고 투자도 늘어나면서 시장 성장률에 새로운 기회가 생길 것입니다.
- 시장 참여자들의 전략적 이니셔티브
CRISPR 유전자 검출 및 진단에 대한 수요는 미국에서 수요를 증가시켰고 만성 질환의 시기적절한 치료로 인해 이러한 유리한 요인은 약물에 대한 필요성을 높이고 시장 수요를 달성하기 위해 소규모 및 대규모 시장 참여자는 다양한 전략을 활용하고 있습니다.
주요 기업들은 또한 사업을 원활하게 운영하고, 위험을 피하고, 시장 판매의 장기적 성장을 높이기 위해 제품 출시, 인수, 승인, 확장, 파트너십 등의 구체적인 전략을 고안하려고 노력하고 있습니다.
예를 들어,
- 2021년 5월, Horizon Discovery Ltd.는 Waltham에서 CRISPR 간섭을 위한 최초의 합성 단일 가이드 RNA와 특허 출원 중인 dcas9 리프레서로 유전자 조절 포트폴리오를 확장했습니다. 포트폴리오 확장으로 미국과 영국 지역 전체에서 합성 가이드 RNA 포트폴리오의 판매와 수익이 증가했으며 시장 참여자와의 협력이 증가했습니다.
또한 효과적인 치료법과 지속적인 임상 시험의 출시는 2022-2029년 예측 기간 동안 CRISPR 유전자 검출 및 진단 시장에 유익한 기회를 제공할 것입니다. 또한, 현재와 의료 기술의 높은 충족되지 않은 요구와 발전은 미래에 CRISPR 유전자 검출 및 진단 시장의 성장률을 가속화할 것입니다.
제약/도전
그러나 CRISPR 진단의 높은 비용과 CRISPR 진단을 사용하는 동안 직면한 위험은 CRISPR 유전자 검출 및 진단 시장의 성장률을 방해할 것입니다. 또한 MRI 장치를 사용하는 동안 발생하는 위험은 CRISPR 유전자 검출 및 진단 시장 성장을 방해할 것입니다. 숙련된 전문 지식과 규정의 부족은 위에서 언급한 예측 기간 동안 시장에 더욱 큰 도전이 될 것입니다.
- CRISPR 기반 진단 비용 상승
CRISPR 기반 치료법의 방대한 잠재력에는 비용 태그가 있습니다. 최대 게놈 편집 치료법은 개발 및 생산에 더 많은 시간이 필요하므로 비용이 상승합니다. 게다가 CRISPR 유전자 검출 및 진단과 관련된 검사 키트와 약물은 많은 인구에 적용 가능합니다. 이러한 비용은 환자에게 부담이 됩니다. 따라서 현재의 높은 비용은 앞으로 감소 추세를 보일 것으로 예상됩니다.
예를 들어,
- Integrated DNA Technologies, Inc.에 따르면 2021년 7월 현재 사전 증폭으로 역전사 LAMP(RT-LAMP)를 포함하는 SARS-CoV-2에 대한 최초의 상용 CRISPR 기반 진단 검사가 반응당 30.15달러에 판매되고 있습니다.
CRISPR 유전자 검출 및 진단 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 창출처, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. CRISPR 유전자 검출 및 진단 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
환자 역학 분석
Globocan의 연구에 따르면, 2020년 유방암은 약 11.7%로 발병률이 높았고, 그 다음으로 폐암이 11.40%, 대장암이 10.00%, 자궁경부암과 식도암이 발병률이 낮았습니다.
CRISPR 유전자 검출 및 진단 시장은 또한 환자 분석, 예후 및 치료에 대한 자세한 시장 분석을 제공합니다. 유병률, 발생률, 사망률, 준수율은 보고서에서 사용할 수 있는 일부 데이터 변수입니다. 시장 성장에 대한 역학의 직접 또는 간접 영향 분석을 분석하여 성장 기간 동안 시장을 예측하기 위한 보다 견고하고 코호트 다변량 통계 모델을 만듭니다.
CRISPR 유전자 검출 및 진단 시장 에 대한 COVID-19 영향
COVID-19는 시장에 부정적인 영향을 미쳤습니다. 팬데믹 동안의 봉쇄와 격리는 진단 관리와 치료를 복잡하게 만듭니다. 일상적이고 약물 투여를 위한 의료 시설에 대한 접근성 부족은 시장에 더 큰 영향을 미칠 것입니다. 사회적 고립은 스트레스, 절망, 사회적 지원을 증가시키며, 이 모든 것이 팬데믹 동안 항경련제 약물 복용을 감소시킬 수 있습니다.
최근 개발
- 2020년 8월, SHERLOCK BIOSCIENCES는 Dartmouth-Hitchcock Health와 협력하여 Sars-CoV-2에 대한 SHERLOCK 진단 키트의 임상 시험을 실시한다고 발표했습니다. 이 키트는 미국 식품의약국(FDA)의 긴급 사용 허가(EUA)로부터 긴급 승인을 받았습니다.
북미 CRISPR 유전자 검출 및 진단 시장 범위
CRISPR 유전자 검출 및 진단 시장은 클래스, 제품 및 서비스, 애플리케이션, 워크플로, 최종 사용자, 유통 채널의 6개 세그먼트를 기준으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 애플리케이션을 식별하기 위한 전략적 결정을 내리는 데 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
수업
- 클래스 1 - 다중 효과 단백질
- 2등급 - 단일 CrRNA 결합 단백질
CRISPR 유전자 검출 및 진단 시장은 클래스를 기준으로 클래스 1(다중 효과 단백질)과 클래스 2(단일 CrRNA 결합 단백질)로 구분됩니다.
제품 및 서비스
- 제품
- 서비스
CRISPR 유전자 검출 및 진단 시장은 제품과 서비스를 기준으로 다음과 같이 세분화됩니다.
애플리케이션
- 생물의학 진단
- 게놈 엔지니어링
- 약물 발견
- 농업 응용 프로그램
- 기타
CRISPR 유전자 검출 및 진단 시장은 응용 분야를 기준으로 생물의학 진단, 유전체 공학, 신약 발견, 농업 응용 분야 등으로 세분화됩니다.
작업 흐름
- 샘플 준비
- 사전 증폭
- 크립RNA
- 카스 효소
- 감지
워크플로우를 기준으로 CRISPR 유전자 검출 및 진단 시장은 샘플 준비, 사전 증폭, CrRNA, Cas 효소 및 감지로 구분됩니다.
최종 사용자
- 병원
- 진단 센터
- 생명공학 회사
- 학술 및 연구 기관
- 기타
최종 사용자를 기준으로 CRISPR 유전자 검출 및 진단 시장은 병원, 진단 센터, 생명공학 기업, 학술 및 연구 기관 등으로 세분화됩니다.
유통 채널
- 직접 입찰
- 소매 판매
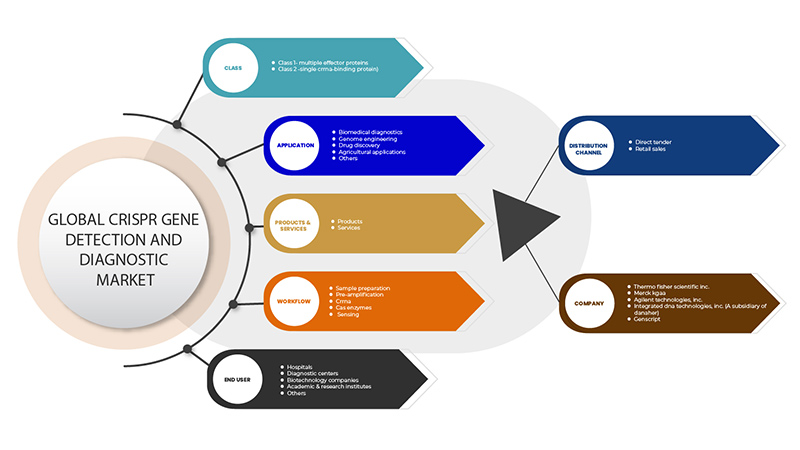
유통 채널을 기준으로 CRISPR 유전자 검출 및 진단 시장은 직접 입찰과 소매 판매로 구분됩니다.
CRISPR 유전자 검출 및 진단 시장 지역 분석/통찰력
The North America CRISPR gene detection and diagnostic market is analysed and market size insights and trends are provided by regions, class, products & services, application, workflow, end user, and distribution channel as referenced above.
The countries covered in the CRISPR gene detection and diagnostic market report are U.S., Canada and Mexico.
The U.S. dominates the CRISPR gene detection and diagnostic market due to the rise in healthcare expenditure.
The country section of the report also provides individual market impacting factors and changes in regulations in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and CRISPR Gene Detection and Diagnostic Market Share Analysis
The North America CRISPR gene detection and diagnostic market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, the North American presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the CRISPR gene detection and diagnostic market.
Some of the major players operating in the CRISPR gene detection and diagnostic market are GenScript, OriGene Technologies, Inc., Applied StemCell, GeneCopoeia, Inc., Agilent Technologies, Inc., Synthego, BioVision Inc., Hera Biolabs, Cellecta, Inc., New England Biolabs, 10x Genomics, addgene CasTag Biosciences, Merck KGaA, Integrated DNA Technologies, Inc. (A subsidiary of Danaher), Thermo Fisher Scientific Inc. among others.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 CLASS SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 INTELLECTUAL PROPERTY LANDSCAPE (PATENT LANDSCAPE)
6 EPIDEMIOLOGY
7 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: REGULATORY SCENARIO
8 PIPELINE ANALYSIS FOR NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, OF CRISPR DIAGNOSTICS
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
9.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT
9.1.3 AVAILABILITY OF FUNDING FOR CRISPR GENE DIAGNOSTICS
9.1.4 RISE IN GMP-CERTIFICATION APPROVALS FOR CRISPR GENE DIAGNOSTIC
9.1.5 RISE IN CLINICAL TRIALS FOR CRISPR BASED DIAGNOSTICS
9.2 RESTRAINTS
9.2.1 RISE IN COST OF CRISPR BASED DIAGNOSTICS
9.2.2 RISKS FACED WHILE USING CRISPR DIAGNOSIS
9.2.3 ETHICAL CONCERNS RELATED TO CRISPR GENE DETECTION AND DIAGNOSTIC RESEARCH
9.2.4 AVAILABILITY OF ALTERNATIVES
9.3 OPPORTUNITIES
9.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
9.3.2 RISE IN HEALTHCARE EXPENDITURE
9.3.3 EMERGENCE OF TECHNOLOGICAL ADVANCEMENTS IN CRISPR BASED DIAGNOSTICS
9.4 CHALLENGES
9.4.1 LACK OF SKILLED PROFESSIONALS REQUIRED FOR CRISPR DIAGNOSTICS
9.4.2 STRINGENT REGULATIONS
10 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS
10.1 OVERVIEW
10.2 CLASS-2 SINGLE CRRNA-BINDING PROTEIN
10.2.1 BIOMEDICAL DIAGNOSTICS
10.2.2 AGRICULTURAL APPLICATIONS
10.2.3 GENOME ENGINEERING
10.2.4 DRUG DISCOVERY
10.2.5 OTHERS
10.3 CLASS-1 MULTIPLE EFFECTOR PROTEINS
11 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES
11.1 OVERVIEW
11.2 PRODUCTS
11.2.1 ASSAY KITS
11.2.1.1 SGRNA KIT
11.2.1.2 GENOMIC DETECTION KIT
11.2.1.3 OTHERS
11.2.2 PROTEINS
11.2.2.1 CAS9
11.2.2.2 CPF1
11.2.2.3 OTHERS
11.2.3 PLASMID AND VECTOR
11.2.4 LIBRARY
11.2.5 CONTROL KITS
11.2.6 DELIVERY SYSTEM PRODUCTS
11.2.7 DESIGN TOOLS
11.2.8 GENOMIC RNA
11.2.9 HDR BLOCKERS
11.2.9.1 AZIDOTHYMIDINE
11.2.9.2 TRIFLUOROTHYMIDINE
11.2.9.3 OTHERS
11.2.9.4 OTHERS
11.3 SERVICES
11.3.1 G-RNA DESIGN
11.3.2 CELL LINE ENGINEERING
11.3.3 MICROBIAL GENE EDITING
11.3.4 DNA SYNTHESIS
11.3.5 OTHERS
12 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION
12.1 OVERVIEW
12.2 BIOMEDICAL DIAGNOSTICS
12.2.1 CANCER
12.2.2 BLOOD DISORDERS
12.2.3 HEREDITARY DISORDERS
12.2.4 MUSCULAR DYSTROPHY
12.2.5 AIDS
12.2.6 NEURODEGENERATIVE CONDITION
12.2.7 OTHERS
12.3 AGRICULTURAL APPLICATIONS
12.4 GENOME ENGINEERING
12.4.1 CELL LINE ENGINEERING
12.4.2 HUMAN STEM CELLS
12.5 DRUG DISCOVERY
12.6 OTHERS
13 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW
13.1 OVERVIEW
13.2 CRRNA
13.3 CAS ENZYME
13.4 PRE-AMPLIFICATION
13.4.1 PCR
13.4.2 LAMP
13.4.3 RPA
13.5 SAMPLE PREPARATION
13.6 SENSING
13.6.1 FLUORESCENT PROBES
13.6.2 COLORIMETRIC
14 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER
14.1 OVERVIEW
14.2 BIOTECHNOLOGY COMPANIES
14.3 ACADEMIC AND RESEARCH INSTITUTES
14.4 DIAGNOSTIC CENTERS
14.5 HOSPITALS
14.6 OTHERS
15 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
16 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 THERMO FISHER SCIENTIFIC INC.
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENTS
19.2 MERCK KGA
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 AGILENT TECHNILOGIES, INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENT
19.4 INTEGRATED DNA TECHNOLOGIES, INC. (A SUBSIDIARY OF DANAHER)
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENTS
19.5 GENSCRIPT
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.6 10 X GENOMICS
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.7 APPLIED STEM CELL
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 ADDGENE
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 BIOVISION INC.
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CELLECTA, INC
19.10.1 COMPANY SNAPSHOT
19.10.2 PRODUCT PORTFOLIO
19.10.3 RECENT DEVELOPMENTS
19.11 CAS TAG BIOSCIENCES
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 GENECOPOEIA, INC.
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 HORIZON DISCOVERY LTD
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENTS
19.14 HERA BIOLABS
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 NEW ENGLAND BIOLABS
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENTS
19.16 ORIGENE TECHNOLOGIES, INC.
19.16.1 COMPANY SNAPSHOT
19.16.2 PRODUCT PORTFOLIO
19.16.3 RECENT DEVELOPMENT
19.17 SYNTHEGO
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENTS
19.18 TAKARA BIO INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 TOOLGEN, INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 PRODUCT PORTFOLIO
19.19.3 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
표 목록
TABLE 1 PIPELINE ANALYSIS FOR NORTH AMERICA CRISPR GENE THERAPEUTICS
TABLE 2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA CLASS-1 MULTIPLE EFFECTOR PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA AGRICULTURAL APPLICATIONS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA DRUG DISCOVERYIN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA CRRNA IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA CAS ENZYME IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SAMPLE PREPARATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA BIOTECHNOLOGY COMPANIES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA ACADEMIC AND RESEARCH INSTITUTES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA DIAGNOSTIC CENTERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA HOSPITALS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA DIRECT TENDER IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA RETAIL SALES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 57 U.S. CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 58 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 59 U.S. PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 U.S. ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.S. HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 62 U.S. PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 63 U.S. SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 65 U.S. GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 66 U.S. BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 68 U.S. PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 69 U.S. SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 70 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 72 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 73 CANADA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 75 CANADA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 CANADA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 77 CANADA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 CANADA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 79 CANADA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 80 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 81 CANADA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 82 CANADA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 84 CANADA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 85 CANADA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 86 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 87 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 88 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 89 MEXICO CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 90 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 91 MEXICO PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 92 MEXICO ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 93 MEXICO HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 94 MEXICO PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 95 MEXICO SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 96 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 97 MEXICO GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 98 MEXICO BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 99 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 100 MEXICO PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 101 MEXICO SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 102 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DBMR POSITION GRID
FIGURE 8 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN TECHNOLOGICAL ADVANCEMENTS IN CRISPR DIAGNOSTICS, AND GOVERNMENT FUNDING FOR THE DEVELOPMENT OF CRISPR DETECTION KITS ARE EXPECTED TO DRIVE THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 TO 2029
FIGURE 13 CLASS SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 & 2029
FIGURE 14 NORTH AMERICA CRISPR GENE PATENT SCENARIO, BY APPLICATION
FIGURE 15 CRISPR PATENT LANDSCAPE AND NUMBER OF APPLICATIONS OF NEW PATENT FAMILIES FILED WORLDWIDE, 2001 TO 2019
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
FIGURE 17 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 18 PREVALENCE OF HUNTINGTON’S DISEASE IN 2019
FIGURE 19 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2021
FIGURE 20 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2022-2029 (USD MILLION)
FIGURE 21 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, CAGR (2022-2029)
FIGURE 22 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, LIFELINE CURVE
FIGURE 23 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2021
FIGURE 24 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2022-2029 (USD MILLION)
FIGURE 25 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, CAGR (2022-2029)
FIGURE 26 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, LIFELINE CURVE
FIGURE 27 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2021
FIGURE 28 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 29 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 30 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 31 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2021
FIGURE 32 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2022-2029 (USD MILLION)
FIGURE 33 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, CAGR (2022-2029)
FIGURE 34 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, LIFELINE CURVE
FIGURE 35 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 36 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 38 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 39 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 44 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 45 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS (2022-2029)
FIGURE 48 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.