Middle East And Africa Adalimumab Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
134.81 Million
USD
181.68 Million
2021
2029
USD
134.81 Million
USD
181.68 Million
2021
2029
| 2022 –2029 | |
| USD 134.81 Million | |
| USD 181.68 Million | |
|
|
|
Middle East and Africa Adalimumab Market, By Drug class (Antirheumatics, TNF Alfa Inhibitors, Others), Indication (Rheumatoid Arthritis, Ankylosing Spondylitis, Chronic Plaque Psoriasis, Crohn's Disease, Ulcerative Colitis, Psoriatic Arthritis, Juvenile Idiopathic Arthritis, Hidradenitis Suppurativa, Non-Infectious Intermediate, Others), Type (Biologics, Biosimilars), Dosage Strength (40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.2mlg, 10mg/0.1mlg, Others), Drug Type (Branded, Generics), Route of Administration (Oral, Parenteral, Others), Age Group (Pediatric, Adult, Geriatric), Dosage Form (Tablet, Injection, Solution, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Market Analysis and Size
Adalimumab, which was first licensed in the United States, is now available in more than 60 countries. Its Middle East and Africa market is consolidated, with only a few firms attempting to outsmart one other on price. Most of the major players are currently concentrating their efforts on the development of adalimumab biosimilars for the treatment of rheumatoid arthritis and psoriasis. This is seen in clinical trials testing the safety and efficacy of adalimumab biosimilars in the treatment of medical disorders. Many inflammatory disorders in adults are treated with adalimumab, including ulcerative colitis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, and hidradenitis suppurativa.
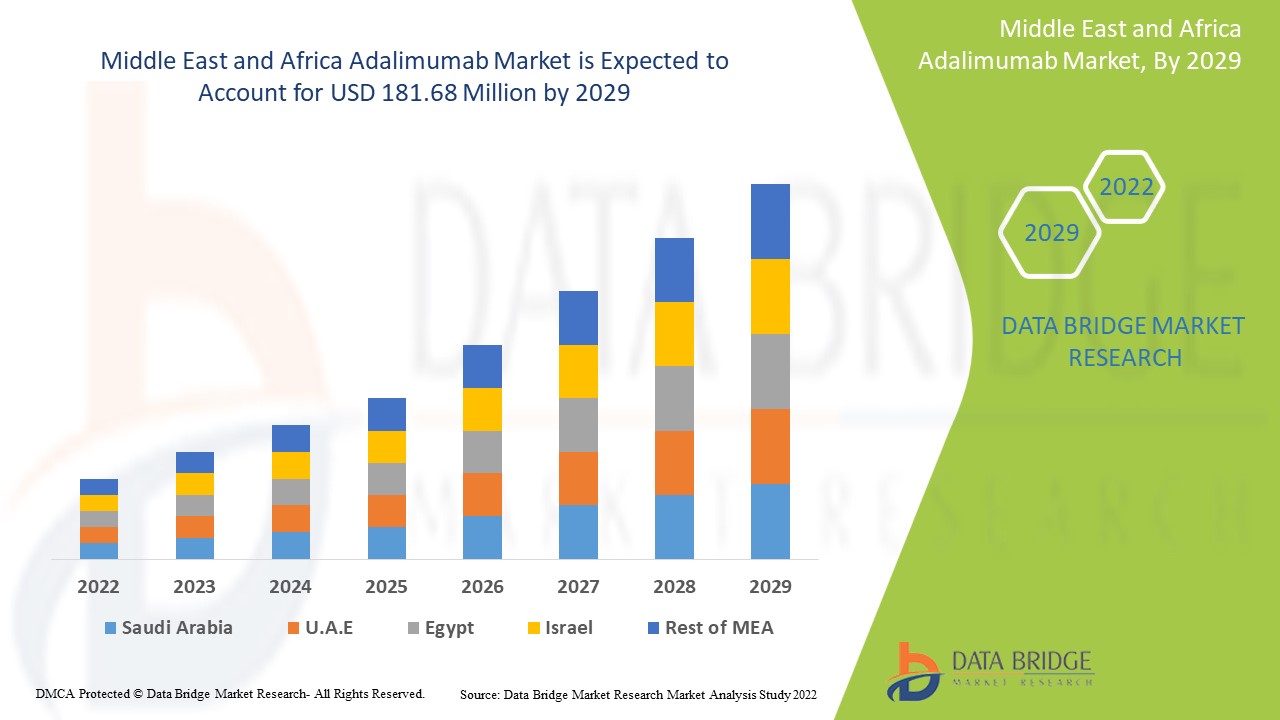
Data Bridge Market Research analyses that the Middle East and Africa adalimumab market was valued at USD 134.81 million in 2021 and is expected to reach USD 181.68 million by 2029, registering a CAGR of 3.80% during the forecast period of 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
약물 종류(항류마티스제, TNF 알파 억제제, 기타), 적응증(류마티스 관절염, 강직성 척추염, 만성 플라크 건선, 크론병, 궤양성 대장염, 건선성 관절염, 소아 특발성 관절염, 농양성 히드라데니티스, 비감염성 중간체, 기타), 유형(생물학적 제제, 바이오시밀러), 용량 강도(40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.2mlg, 10mg/0.1mlg, 기타), 약물 종류(브랜드, 제네릭), 투여 경로(경구, 비경구, 기타), 연령대(소아, 성인, 노인), 투여 형태(정제, 주사, 용액, 기타), 최종 사용자(병원, 전문 병원, 재택 치료, 기타), 유통채널(병원약국, 소매약국, 온라인약국, 기타) |
|
적용 국가 |
사우디아라비아, UAE, 남아프리카공화국, 이집트, 이스라엘, 중동 및 아프리카(MEA)의 일부인 기타 중동 및 아프리카(MEA) |
|
시장 참여자 포함 |
Mylan NV(미국), AbbVie Inc.(미국), Zydus Cadila(인도), Pfizer Inc.(미국), Hetero Biopharma Ltd.(인도), Boehringer Ingelheim International GmbH.(독일) |
|
시장 기회 |
|
시장 정의
아달리무맙은 Humira와 Exemptia라는 브랜드 이름으로 판매되는 처방약입니다. 혈우마티스 관절염, 건선성 관절염, 크론병, 건선 및 궤양성 대장염은 모두 아달리무맙으로 치료합니다. TNF(종양괴사인자 알파)는 일반적으로 아달리무맙에 의해 결합됩니다. TNF가 TBF 수용체와 상호 작용하면 자가면역 질환에 대한 염증 반응이 유발됩니다. 아달리무맙은 TNF에 결합함으로써 염증 반응의 가능성을 줄입니다.
중동 및 아프리카 아달리무맙 시장 동향
운전자
- 자가면역질환 발병률 증가
건선성 관절염, 판상 건선, 궤양성 대장염, 강직성 척추염, 류마티스 관절염 및 크론병과 같은 자가면역 질환의 발생률 증가는 시장 성장률을 크게 높일 것으로 예상됩니다. 아달리무맙은 통증과 부기를 줄이는 동시에 관절염의 진행을 늦추는 약물입니다. 아달리무맙은 활성 엔테시스 관련 관절염, 류마티스 관절염, 골관절염, 다관절 소아 특발성 관절염 및 기타 자가면역 질환을 치료하는 데 사용됩니다. 이와 함께 만성 질환의 유병률이 증가함에 따라 아달리무맙 시장 수요가 증가할 것입니다.
- 의료 인프라에 대한 투자 증가
아달리무맙 시장의 성장률에 영향을 미치는 또 다른 중요한 요인은 인프라를 개선하는 데 도움이 되는 의료비 지출 증가입니다. 또한, 다양한 정부 기관은 자금을 늘려 의료 인프라를 개선하고자 하며, 이는 시장 역학에 더욱 영향을 미칠 것입니다.
- 피부 질환 발생률 증가
피부 질환의 발생률은 2022-2029년 예측 기간 동안 시장 성장률을 촉진할 것으로 추산됩니다. 세계보건기구(WHO)는 전 세계적으로 9억 명이 언제나 피부 질환을 앓고 있다고 추정합니다. TNF-알파(종양괴사인자-알파)는 건선을 포함한 피부 질환을 일으키는 염증 과정에 중요한 참여자입니다. 아달리무맙은 신체에서 이 단백질을 표적으로 삼습니다. 건선은 무릎, 팔꿈치, 몸통, 두피에 비늘 같은 붉은 반점이 생기는 피부 질환입니다. 건선은 아달리무맙에 의해 억제되는 과활성 면역 체계 반응으로 인해 발생합니다. National Psoriasis Foundation에 따르면 전 세계적으로 1억 2,500만 명이 건선을 앓고 있으며, 이는 전체 인구의 2~3%를 차지하여 시장 성장을 촉진합니다.
또한, 공공 및 민간 기관에서 바이오시밀러 약물에 대한 인식을 확산하기 위한 이니셔티브가 증가하고 비용 효율성으로 인해 바이오시밀러 약물에 대한 수요가 급증함에 따라 아달리무맙 시장이 확대될 것입니다. 또한, 노령 인구의 급증과 상부 호흡기 감염 사례의 증가로 인해 아달리무맙 시장이 확대될 것입니다.
기회
- 연구개발 활동의 증가
게다가, 시장 성장은 연구 개발 활동의 증가에 의해 촉진됩니다. 이는 아달리무맙 시장 성장에 유익한 기회를 제공할 것입니다. 이와 함께, 약물 승인 및 출시 증가는 시장 성장률을 더욱 촉진할 것입니다.
게다가 첨단 기술 개발을 위한 투자가 증가하고 신흥 시장의 수가 늘어나면서 예측 기간 동안 아달리무맙 시장 성장에 유익한 기회가 더욱 제공될 것입니다.
제약/도전
- 아달리무맙 과 관련된 높은 비용과 부작용
아달리무맙은 저소득 및 중소득 국가의 사람들에게는 매우 비싸며, 주입당 약 2,000~3,000달러가 듭니다. 더욱이 아달리무맙의 부정적 효과는 시장 확장을 제한할 것으로 예상됩니다. 발열, 샘 부기, 야간 발한, 전반적인 불쾌감, 관절 및 근육 통증, 피부 발진, 쉽게 멍이 들거나 출혈하는 것 등이 아달리무맙의 흔한 부작용 중 일부입니다. 아달리무맙은 또한 치명적인 림프종과 간암, 비장암 및 골수암을 일으킬 수 있습니다. 이는 크론병이나 궤양성 대장염이 있는 청소년과 젊은 남성에게 가장 흔하여 시장 성장을 늦춥니다.
반면, 개발도상국의 의료 인프라 부족과 바이오시밀러 제품 승인과 관련된 엄격한 규제 절차는 아달리무맙 시장에 도전이 될 것입니다. 또한, 약물의 특허 만료는 2022-2029년 예측 기간 동안 시장 성장률을 제한하고 더욱 방해할 것입니다.
이 중동 및 아프리카 아달리무맙 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 창출처, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 중동 및 아프리카 아달리무맙 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
환자 역학 분석
중동 및 아프리카 아달리무맙 시장은 또한 환자 분석, 예후 및 치료법에 대한 자세한 시장 분석을 제공합니다. 유병률, 발생률, 사망률, 준수율은 보고서에서 사용할 수 있는 일부 데이터 변수입니다. 시장 성장에 대한 역학의 직접 또는 간접 영향 분석을 분석하여 성장 기간 동안 시장을 예측하기 위한 보다 견고하고 코호트 다변량 통계 모델을 만듭니다.
COVID-19 중동 및 아프리카 Adalimumab 시장 에 미치는 영향
2019년 12월에 출현한 이래 COVID-19 바이러스는 지구상 거의 모든 국가로 퍼져 세계보건기구(WHO)가 이를 공중보건 비상사태로 선언하게 되었습니다. 새로운 코로나바이러스인 COVID-19는 폐렴 사례의 원인균으로 확인되었습니다. 이 바이러스는 전 세계로 빠르게 퍼져 많은 사람을 죽였습니다. COVID-19는 2020년 3월에 세계보건기구(WHO)에서 중동 및 아프리카 팬데믹으로 지정되었으며, 이 질병의 확산을 막기 위한 엄격한 조치가 권장되었습니다. 그 이후로 이 팬데믹은 의료 부문의 확장을 지연시키고 공급망을 혼란에 빠뜨렸습니다. 게다가 여러 국가의 정부는 COVID-19의 확산을 막기 위해 전국적인 봉쇄령을 내렸습니다. 마찬가지로 전 세계 여러 국가의 의료 기관은 공급망 활동을 계속하는 데 어려움을 겪고 있었습니다. 아달리무맙 시장은 공급망 둔화로 어려움을 겪었습니다.
최근 개발
- 2021년 10월, 미국 식품의약국(FDA)은 다양한 염증성 질환 치료를 위한 최초의 상호 교환형 바이오시밀러 제품의 승인을 발표했습니다. 바이오시밀러 및 상호 교환형 승인 경로는 중증 질환을 앓고 있는 환자가 더 많은 치료 옵션을 이용할 수 있도록 돕기 위해 마련되었습니다. Cyltezo는 FDA가 승인한 최초의 상호 교환형 단일클론 항체이자 두 번째 상호 교환형 바이오시밀러 의약품입니다.
중동 및 아프리카 Adalimumab 시장 범위
중동 및 아프리카 아달리무맙 시장은 약물 종류, 유형, 적응증, 투여 형태, 투여 강도, 약물 유형, 투여 경로, 연령대, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 귀중한 시장 개요와 시장 통찰력을 제공하여 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내리는 데 도움이 됩니다.
약물 종류
- 항류마티스제
- TNF 알파 억제제
- 기타
표시
- 류머티스성 관절염
- 강직성 척추염
- 만성 플라크 건선
- 크론병
- 궤양성 대장염
- 건선성 관절염
- 청소년 특발성 관절염
- 농양성 히드라데니티스
- 비감염성 중간체
- 기타
유형
- 생물학
- 바이오시밀러
복용량 강도
- 40mg/0.4mlg
- 80mg/0.8mlg
- 20mg/0.2mlg
- 10mg/0.1mlg
- 기타
약물 유형
- 브랜드화
- 제네릭
투여 경로
- 경구
- 비경구적
- 기타
투여 형태
- 주입
- 해결책
- 태블릿
- 기타
연령대
- 소아과
- 성인
- 노인
최종 사용자
- 병원
- 전문 클리닉
- 홈케어
- 기타
유통 채널
- 병원 약국
- 소매 약국
- 온라인 약국
- 기타
중동 및 아프리카 아달리무맙 시장 지역 분석/통찰력
중동 및 아프리카 아달리무맙 시장을 분석하고, 위에 언급된 대로 국가, 약물 종류, 유형, 적응증, 투여 형태, 투여 강도, 약물 유형, 투여 경로, 연령대, 최종 사용자 및 유통 채널별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
중동 및 아프리카 아달리무맙 시장 보고서에서 다루는 국가는 사우디아라비아, UAE, 남아프리카공화국, 이집트, 이스라엘, 중동 및 아프리카(MEA)의 일부인 기타 중동 및 아프리카(MEA)입니다.
사우디아라비아는 이 지역의 관절염 질환 부담을 극복하기 위한 연구 개발 활동이 늘어나면서 아달리무맙 시장을 장악하고 있습니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변화를 제공합니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 트렌드 및 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 중동 및 아프리카 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 중동 및 아프리카 Adalimumab 시장 점유율 분석
The Middle East and Africa adalimumab market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Middle East and Africa presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Middle East and Africa adalimumab market.
Some of the major players operating in the Middle East and Africa adalimumab market are:
- Mylan N.V. (US)
- Zydus Cadila (India)
- Boehringer Ingelheim International GmbH. (Germany)
- AbbVie Inc. (US)
- Abbott (US)
- Hetero Biopharma Ltd. (India)
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2 MG/0.4ML
13.3 MG/0.8ML
13.4 MG/0.4ML
13.5 MG/0.1ML
13.6 OTHERS
14 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 MIDDLE EAST & AFRICA
19.1.1 SAUDI ARABIA
19.1.2 SOUTH AFRICA
19.1.3 UAE
19.1.4 ISRAEL
19.1.5 KUWAIT
19.1.6 EGYPT
19.1.7 REST OF MIDDLE EAST & AFRICA
20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
표 목록
LIST OF TABLES
TABLE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 SAUDI ARABIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 SAUDI ARABIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 SAUDI ARABIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 SAUDI ARABIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 SAUDI ARABIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 SAUDI ARABIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 SAUDI ARABIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 SAUDI ARABIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 SAUDI ARABIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 SAUDI ARABIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 SOUTH AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 SOUTH AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 SOUTH AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 SOUTH AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 SOUTH AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 SOUTH AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 SOUTH AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 SOUTH AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 SOUTH AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 SOUTH AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 UAE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 UAE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 UAE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 UAE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 UAE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 UAE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 UAE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 UAE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 UAE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 UAE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 ISRAEL ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 ISRAEL ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 ISRAEL BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 ISRAEL ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 ISRAEL ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 ISRAEL GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 ISRAEL ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 ISRAEL ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 ISRAEL ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 ISRAEL ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 KUWAIT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 KUWAIT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 KUWAITBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 KUWAIT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 KUWAIT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 KUWAIT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 KUWAIT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 KUWAIT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 KUWAIT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 KUWAIT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 EGYPT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 EGYPT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 EGYPT BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 EGYPT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 EGYPT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 EGYPT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 EGYPT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 EGYPT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 EGYPT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 EGYPT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 REST OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
그림 목록
LIST OF FIGURES
FIGURE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.