Global Transcatheter Pulmonary Valve Replacement Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
66.69 Million
USD
116.57 Million
2024
2032
USD
66.69 Million
USD
116.57 Million
2024
2032
| 2025 –2032 | |
| USD 66.69 Million | |
| USD 116.57 Million | |
|
|
|
|
Global Transcatheter Pulmonary Valve Replacement Market Segmentation, By Product Type (Balloon-Expandable Valves and Self-Expandable Valves), Material (Biological Valves and Synthetic Valves), Indication (Pulmonary Valve Stenosis and Pulmonary Valve Regurgitation), End-User (Hospitals, Ambulatory Surgical Centers (ASCs), and Specialty Clinics) – Industry Trends and Forecast to 2032
Transcatheter Pulmonary Valve Replacement Market Analysis
The global transcatheter pulmonary valve replacement (TPVR) market is experiencing significant growth, driven by the increasing prevalence of congenital heart diseases (CHDs), which affect approximately 1 in 100 live births worldwide. In the U.S., 40,000 babies are born with CHDs each year, with about 25% requiring early medical interventions to manage the condition. As the global population ages, there is a rise in valve degeneration cases, further emphasizing the need for effective treatments. TPVR procedures, which offer a minimally invasive alternative to traditional open-heart surgeries, are gaining popularity due to their ability to reduce risks, shorten recovery times, and improve patient outcomes. These advancements in less invasive options are expanding the adoption of TPVR, positioning it as a preferred solution for treating both congenital and age-related heart valve conditions.
Transcatheter Pulmonary Valve Replacement Market Size
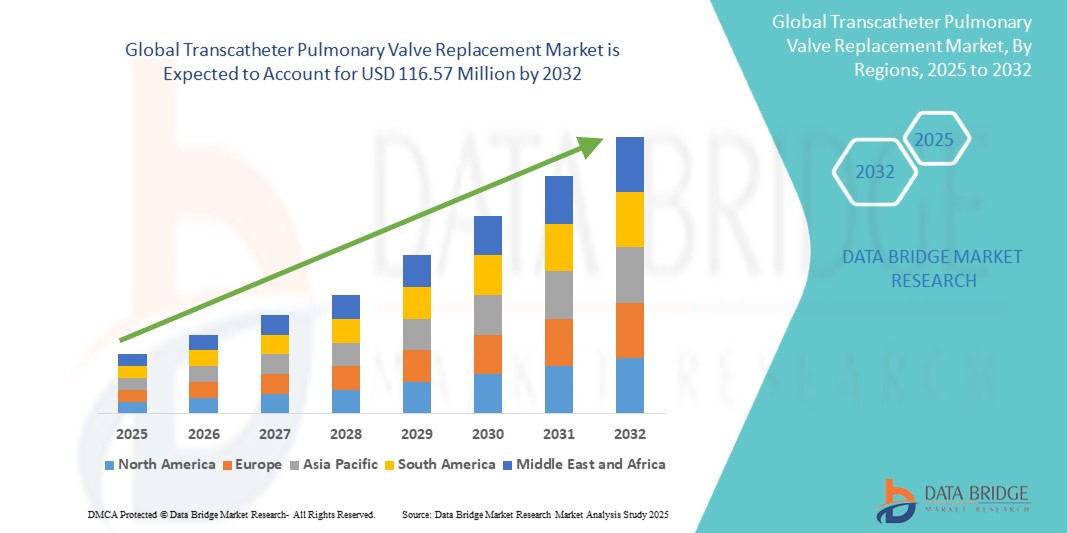
Global transcatheter pulmonary valve replacement market size was valued at USD 66.69 million in 2024 and is projected to reach USD 116.57 million by 2032, with a CAGR of 7.23% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Transcatheter Pulmonary Valve Replacement Market Trends
“Growing Acceptance in Pediatric and Adult Populations”
The growing acceptance of Transcatheter pulmonary valve replacement (TPVR) in both pediatric and adult populations is emerging as a notable trend in the market. While TPVR was initially used primarily in pediatric patients with congenital heart defects, its application is expanding to adult populations, especially those with valve degeneration or failure. This shift reflects a broader recognition of TPVR’s benefits in treating pulmonary valve issues in adults who have undergone previous surgeries or experienced valve complications over time. As more patients, both young and older, benefit from this less invasive option, TPVR is becoming a widely accepted treatment for a broader range of conditions, contributing to its increasing use across diverse age groups.
Report Scope and Transcatheter Pulmonary Valve Replacement Market Segmentation
|
Attributes |
Transcatheter Pulmonary Valve Replacement Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Medtronic (Ireland), Edwards Lifesciences Corporation (U.S.), JenaValve Technology, Inc. (Germany), Boston Scientific Corporation (U.S.), Abbott (U.S.), Terumo Corporation (Japan), Rudolf Medical GmbH (Germany), LivaNova PLC (UK), MedeAnalytics, Inc. (U.S.), CryoLife, Inc. (U.S.), Biotronik SE & Co. KG (Germany), Vascular Solutions, Inc. (U.S.), Xeltis (Netherlands), Tendyne, Inc. (U.S.), Admedes Schuessler GmbH (Germany), LivaNova PLC (UK), among others. |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Transcatheter Pulmonary Valve Replacement Market Definition
Transcatheter pulmonary valve replacement (TPVR) is a minimally invasive medical procedure used to treat patients with pulmonary valve dysfunction, typically caused by pulmonary valve stenosis or regurgitation. In TPVR, a replacement valve is implanted into the pulmonary valve position via a catheter inserted through a blood vessel, usually in the groin, rather than through open-heart surgery. This procedure is commonly used in patients with congenital heart defects or valve degeneration, offering a less invasive option for valve replacement with shorter recovery times and fewer complications.
Transcatheter Pulmonary Valve Replacement Market Dynamics
Drivers
- Rising Prevalence of Congenital Heart Diseases
The rising prevalence of congenital heart diseases (CHDs) is a significant driver for the demand for Transcatheter Pulmonary Valve Replacement (TPVR) procedures. CHDs are among the most common birth defects, affecting approximately 1 in 100 live births globally. Pulmonary valve dysfunction, often due to congenital conditions such as Tetralogy of Fallot (TOF), leads to the need for valve replacement procedures. As more children with these conditions survive into adulthood due to medical advancements, they often experience complications such as pulmonary valve failure or stenosis, necessitating interventions such as TPVR. The ability to offer a minimally invasive alternative to traditional open-heart surgery, particularly for these young and adult patients, significantly increases the adoption of TPVR. Additionally, the growing awareness of early detection and treatment options for congenital heart defects has further fueled the demand for these advanced procedures, ensuring better quality of life and long-term outcomes for patients. For instance, in December 2024, according to an article published by ScienceDirect, the global prevalence of congenital heart diseases (CHDs) in infants is rising each year, with ventricular and atrial septal defects being the most common, occurring more frequently in male infants than female infants. This increasing prevalence is expected to drive demand for advanced treatment options, such as transcatheter pulmonary valve replacement (TPVR) procedures, to address the growing patient population.
- Advancements in Minimally Invasive Technologies
Advancements in minimally invasive technologies are playing a crucial role in the growth of the Transcatheter Pulmonary Valve Replacement (TPVR) market. Technological innovations in TPVR devices, such as balloon-expandable and self-expandable valves, and improvements in catheter delivery systems have made the procedure safer, more efficient, and less invasive than traditional open-heart surgeries. These advancements allow physicians to perform valve replacements through a catheter inserted via a small incision, often in the groin, reducing the need for large surgical cuts and minimizing the risks associated with open surgery. As a result, patients benefit from shorter recovery times, fewer complications, and a reduced risk of infection. These technological innovations also contribute to the broader adoption of TPVR, as they offer better clinical outcomes with less trauma, making the procedure more appealing to both patients and healthcare providers. Consequently, these developments continue to fuel market growth by expanding the patient pool and increasing procedural success rates. In August 2024, according to an article published by BioMed Central Ltd, minimally invasive techniques are becoming more widely adopted worldwide. This growing preference for less invasive procedures is expected to drive the demand for advanced treatments such as transcatheter pulmonary valve replacement (TPVR), offering patients safer and more efficient alternatives to traditional surgery.
Opportunities
- Development of Next-Generation TPVR Devices
The development of next-generation TPVR devices presents a significant opportunity for the growth of the TPVR market. These advanced devices are being designed with improved durability, biocompatibility, and precision to enhance patient outcomes. Innovations in materials, such as more robust and longer-lasting valve components, aim to reduce the frequency of valve failure and the need for repeat interventions. Additionally, advancements in valve design, including better sizing options and more precise placement mechanisms, help reduce procedural complications and improve the success rates of surgeries. As these next-generation devices offer longer valve longevity and fewer risks, they expand the patient pool eligible for TPVR procedures, including those with more complex cases. The continued evolution of these devices not only improves clinical outcomes but also makes the procedure more attractive to both patients and healthcare providers. This, in turn, will accelerate the adoption of TPVR across diverse patient populations. For instance, in February 2024, TPVR has seen significant advancements in device development, evidence-based research, and clinical experience. These improvements are expected to create opportunities for further adoption and growth, as enhanced devices and proven efficacy encourage more widespread use in treating patients with pulmonary valve issues.
- Increasing Collaborations and Partnerships
Collaborations and partnerships between device manufacturers, healthcare providers, and research institutions offer substantial opportunities for the growth of the TPVR market. By working together, these stakeholders can accelerate the development of innovative solutions that address the evolving needs of patients and healthcare systems. Device manufacturers can gain access to the latest research and clinical insights from healthcare providers and institutions, enabling them to improve product offerings and enhance the design of TPVR devices. Additionally, partnerships can foster the development of new technologies, such as better delivery systems and more effective valve materials, which improve patient outcomes and procedural success. In emerging markets, where access to advanced cardiac treatments may be limited, collaborations can help expand market reach by improving the availability and affordability of TPVR devices. These partnerships also create opportunities for training, education, and awareness campaigns, driving adoption and ultimately contributing to the market’s global expansion.
Restraints/Challenges
- High Cost of the TPVR Procedure and Devices
The high cost of the procedure and devices is a significant restraint in the TPVR market. TPVR involves advanced, specialized equipment, including high-quality transcatheter valves and precision delivery systems, all of which come at a premium price. The procedure also requires skilled medical personnel and sophisticated technologies, further increasing its cost. In many regions, especially low-resource settings, these expenses can be prohibitive, limiting the accessibility of TPVR for a large number of patients. Moreover, healthcare reimbursement policies in some countries may not fully cover the costs of such advanced treatments, further restricting patient access. As a result, patients in these areas may have to forgo TPVR or opt for less expensive, but less effective, alternatives, which hinders the adoption of this procedure. The financial burden associated with TPVR procedures thus limits the market’s expansion, particularly in emerging markets and regions with budget constraints. In June 2024, according to an article published by BioMed Central Ltd, the cost of TPVR with commercially available devices such as the Melody valve exceeds that of traditional RVOT surgery. This higher cost is expected to act as a restraint, limiting the adoption of TPVR, particularly in regions with budget constraints or limited reimbursement options.
- Complexity of Procedure and Skill Requirements
The complexity of the TPVR procedure and skill requirements pose a significant challenge to the widespread adoption of this treatment. TPVR is a specialized, highly technical procedure that involves the precise placement of a pulmonary valve using a catheter, often through a minimally invasive approach. This requires experienced cardiologists and surgical teams with expertise in both transcatheter techniques and the nuances of valve replacement in congenital heart disease patients. The procedure demands significant training, and not all healthcare providers are equipped with the necessary skills or infrastructure to perform it. As a result, there may be a shortage of qualified professionals, particularly in regions with limited access to advanced medical training or healthcare facilities. This can restrict the availability of TPVR, particularly in rural or underserved areas, limiting access for patients who could benefit from this advanced treatment. Consequently, this complexity can hinder the global expansion of the TPVR market. In August 2024, according to an article published by ScienceDirect, Transcatheter pulmonary valve replacement (TPVR) can be challenging for patients who lack sufficient femoral or internal jugular vascular access. This limitation is expected to act as a challenge, restricting the procedure's applicability and potentially preventing some patients from receiving this treatment.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Transcatheter Pulmonary Valve Replacement Market Scope
The market is segmented on the basis of product type, material, indication, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Balloon-Expandable Valves
- Self-Expandable Valves
Material
- Biological Valves
- Synthetic Valves
Indication
- Pulmonary Valve Stenosis
- Pulmonary Valve Regurgitation
End-User
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
Transcatheter Pulmonary Valve Replacement Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, product type, material, indication, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its advanced healthcare infrastructure, which enables the widespread availability and adoption of cutting-edge medical technologies. The region's high adoption of minimally invasive procedures, driven by their advantages such as reduced recovery time and lower complication rates, further boosts the demand for TPVR as a safer treatment option.
Asia-Pacific is expected to be the fastest growing increasing prevalence of congenital heart diseases (CHDs), which is becoming more evident in many countries across the region. Rising healthcare investments, particularly in emerging economies, are improving access to advanced medical technologies and specialized treatments.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Transcatheter Pulmonary Valve Replacement Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Transcatheter Pulmonary Valve Replacement Market Leaders Operating in the Market Are:
- Medtronic (Ireland)
- Edwards Lifesciences Corporation (U.S.)
- JenaValve Technology, Inc. (Germany)
- Boston Scientific Corporation (U.S.)
- Abbott (U.S.)
- Terumo Corporation (Japan)
- Rudolf Medical GmbH (Germany)
- LivaNova PLC (UK)
- MedeAnalytics, Inc. (U.S.)
- CryoLife, Inc. (U.S.)
- Biotronik SE & Co. KG (Germany)
- Vascular Solutions, Inc. (U.S.)
- Xeltis (Netherlands)
- Tendyne, Inc. (U.S.)
- Admedes Schuessler GmbH (Germany)
- LivaNova PLC (UK)
Latest Developments in Global Transcatheter Pulmonary Valve Replacement Market
- In May 2024, Edwards Lifesciences has launched the SAPIEN 3 Ultra RESILIA valve in Europe, featuring the company’s innovative RESILIA tissue technology to improve valve durability. This launch strengthens Edwards Lifesciences' competitive edge by offering a more durable solution in the growing transcatheter heart valve market
- In January 2024, Venus Medtech announced that its innovative transcatheter pulmonic valve replacement (TPVR) system, VenusP-Valve, has received approval from Health Canada. This approval enhances the company’s market presence and opens up new growth opportunities in the Canadian healthcare market
- In February 2023, Medtronic has reintroduced its Harmony™ Transcatheter Pulmonary Valve (TPV) System, offering a minimally invasive option for congenital heart disease patients with native or repaired right ventricular outflow tract (RVOT). This relaunch is expected to enhance the company's position in the growing TPVR market by providing a more effective treatment alternative
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.