Global Peptide Api Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
7.89 Billion
USD
16.07 Billion
2024
2032
USD
7.89 Billion
USD
16.07 Billion
2024
2032
| 2025 –2032 | |
| USD 7.89 Billion | |
| USD 16.07 Billion | |
|
|
|
|
Global Peptide API Market Segmentation, By Product Type (Hormonal Peptides, Cyclic Peptides, Linear Peptides, Peptide Derivatives), Therapeutic Area (Oncology, Diabetes, Cardiovascular, Central Nervous System (CNS), Infectious Diseases, Others), Route of Administration (Injectables, Oral, Others), Source (Natural Sources, Synthetic Sources), Application (Research and Development, Commercial Production) – Industry Trends and Forecast to 2032
Peptide API Market Analysis
The global peptide API market is driven by the increasing prevalence of chronic diseases, including cancer, diabetes, and cardiovascular disorders, which are among the leading causes of morbidity and mortality worldwide. According to the International Diabetes Federation (IDF), approximately 537 billion adults aged 20-79 were living with diabetes in 2021, and this number is projected to rise to 783 billion by 2045, creating significant demand for peptide-based therapeutic APIs like glucagon-like peptide-1 (GLP-1) analogs. Additionally, the World Health Organization (WHO) reports that nearly 10 billion deaths in 2020 were attributed to cancer, underlining the need for innovative peptide-based anticancer drugs. The increasing adoption of peptides in targeted therapies due to their high specificity and safety profile further accelerates their application in addressing these rising healthcare burdens.
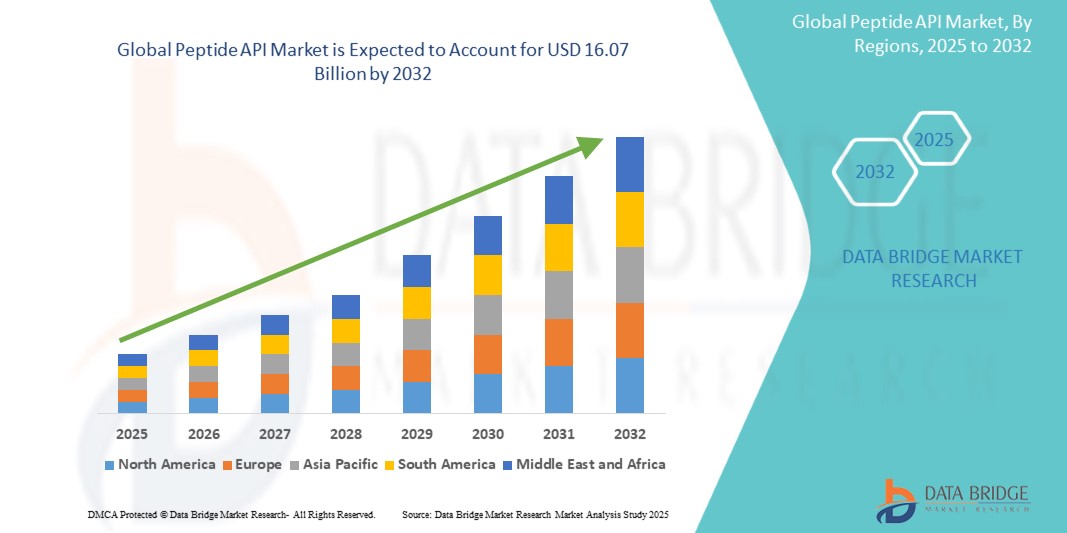
Peptide API Market Size
Global peptide API market size was valued at USD 7.89 billion in 2024 and is projected to reach USD 16.07 billion by 2032, with a CAGR of 9.30% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Peptide API Market Trends
“Advancements in Peptide Synthesis Technologies”
Advancements in peptide synthesis technologies have streamlined the production process, improving efficiency and scalability while maintaining high purity levels. Techniques such as solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS) have been enhanced with automation and hybrid approaches, enabling the production of complex and long-chain peptides with greater precision. Innovations like microwave-assisted peptide synthesis and flow chemistry have further reduced synthesis time and minimized waste, addressing challenges associated with traditional batch production. These advancements support the development of more sophisticated peptide-based APIs, facilitating their application in areas such as oncology, metabolic disorders, and rare diseases.
Report Scope and Peptide API Market Segmentation
|
Attributes |
Peptide API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Novo Nordisk A/S (Denmark), Bachem Holding AG (Switzerland), AstraZeneca (UK), Merck KGaA (Germany), Amgen Inc. (U.S.), H. Lundbeck A/S (Denmark), PeptiDream Inc. (Japan), PolyPeptide Group (France), Thermo Fisher Scientific Inc. (U.S.), AmbioPharm, Inc. (U.S.), CordenPharma International GmbH (Germany), Lonza Group Ltd. (Switzerland), CSBio Company, Inc. (U.S.), ChemPartner Co., Ltd. (China), Dr. Reddy's Laboratories Ltd. (India), Teva Pharmaceutical Industries Ltd. (Israel), Ferring Pharmaceuticals (Switzerland), Pfizer Inc. (U.S.), among others. |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Peptide API Market Definition
Peptide API (Active Pharmaceutical Ingredient) refers to a biologically active compound composed of short chains of amino acids (peptides) that are used in the formulation of therapeutic drugs. Peptide APIs are designed to interact with specific biological targets in the body to produce a desired therapeutic effect. They can be utilized in various therapeutic areas, including oncology, diabetes, cardiovascular diseases, and autoimmune disorders. Peptide APIs mimic naturally occurring biological molecules, offering high specificity and fewer side effects compared to traditional small molecule drugs, which makes them essential components of modern pharmaceuticals.
Peptide API Market Dynamics
Drivers
- Increasing Prevalence of Chronic Diseases
The rising incidence of chronic conditions such as diabetes, cancer, and cardiovascular diseases significantly drives the demand for effective peptide-based therapeutics. As these diseases become more prevalent due to aging populations and unhealthy lifestyles, a larger patient base seeks targeted treatment options. Peptides offer the advantage of specificity, allowing them to interact with specific receptors and minimize damage to healthy cells, which is particularly important in cancer therapy. Many peptide therapeutics, such as insulin for diabetes management, have demonstrated efficacy in managing these chronic conditions while often exhibiting improved safety profiles compared to traditional small molecule drugs.
For instance,
- In March 2022, according to an article published by National Institutes of Health, approximately 21% of the elderly population in India are reported to have at least one chronic disease, with 17% of seniors in rural areas and 29% in urban areas affected. Hypertension and diabetes represent around 68% of these chronic conditions
This growing reliance on peptide-based therapeutics underscores their critical role in addressing the increasing prevalence of chronic conditions. Their specificity, efficacy, and improved safety profiles position peptides as indispensable tools in modern medicine, catering to the evolving needs of a diverse and expanding patient population.
- Ongoing Research and Development
The pharmaceutical and biotechnology industries are significantly investing in the research and development of peptide-based drugs aimed at treating chronic diseases, reflecting a strong commitment to innovation in healthcare. This concentrated focus on developing new peptide therapeutics is driven by the increasing prevalence of chronic conditions such as diabetes, hypertension, and cancer, which demand effective treatment solutions. As these industries advance their R&D efforts, they are likely to discover and develop novel peptide drugs that can provide targeted and more effective treatment options compared to existing therapies. This introduction of innovative peptide-based treatments not only addresses the unmet medical needs of patients but also stimulates market demand as healthcare providers and patients seek out these advanced therapies. Consequently, the ongoing investment and emphasis on peptide drug development are expected to significantly contribute to the growth of the global Peptide API Market.
For instance,
- In October 2023, according to an article published by National Institutes of Health, the therapeutic applications of peptides are an increasingly popular research area, evidenced by the approval of 26 peptide drugs by the Food and Drug Administration (FDA) from 2016 to 2022, amidst a total of 315 new drug approvals during that period. Additionally, there are over 200 peptides currently in clinical development and around 600 undergoing preclinical studies
The ongoing investment in peptide drug research and development highlights the commitment of the pharmaceutical and biotechnology sectors to addressing unmet medical needs. This focus on innovation is poised to expand the therapeutic landscape, offering advanced and targeted treatment options that enhance patient outcomes and further propel the growth of the global Peptide API market.
Opportunities
- Advancements in Technology
Innovations in peptide synthesis and purification technologies are revolutionizing the production of peptide APIs by significantly improving efficiency and scalability. Automated synthesis platforms enable the rapid and precise assembly of peptide chains, reducing the time and labor involved in the synthesis process. This automation minimizes human error, enhances reproducibility, and allows for the production of complex peptides that may be challenging to synthesize manually. Additionally, advancements in purification methods, such as high-performance liquid chromatography (HPLC) and membrane filtration techniques, facilitate the efficient separation and purification of peptides from impurities and by-products. These enhanced purification processes not only increase yield and purity but also reduce production costs and time. As a result, pharmaceutical companies can scale up production to meet growing market demand for peptide therapeutics while ensuring consistent quality.
- Strategic Collaborations and Partnerships
Collaborations between pharmaceutical companies and biotechnology firms play a crucial role in accelerating research and development (R&D) efforts, particularly in the field of peptide therapeutics. By pooling resources, expertise, and technology, these partnerships enable both parties to leverage each other's strengths and capabilities, significantly enhancing the innovation process. Pharmaceutical companies often possess robust infrastructure, regulatory knowledge, and extensive market access, while biotechnology firms typically bring cutting-edge research, specialized technologies, and novel peptide designs to the table. This synergy allows for streamlined R&D processes, where the challenges of peptide synthesis, optimization, and clinical trials can be addressed more efficiently. Additionally, collaborations can lead to shared funding, reducing financial risks associated with developing new therapies. The combined expertise accelerates the discovery and development timelines, facilitating faster market entry for new peptide therapeutics that can address unmet medical needs. As a result, these strategic alliances not only foster innovation but also enhance competitiveness in the rapidly evolving pharmaceutical environment.
For instance,
- In April 2024, CordenPharma and GENEPEP marked one year of collaboration, which began in 2023, to support biotech companies in peptide discovery, development, and early clinical manufacturing. The partnership combines CordenPharma’s cGMP manufacturing, regulatory expertise, and market access with GENEPEP’s drug development and discovery capabilities
Strategic collaborations and partnerships offer a transformative opportunity for advancing peptide therapeutics by combining the strengths of pharmaceutical companies and biotechnology firms. This collaborative approach accelerates innovation, reduces development risks, and enables faster market introduction of novel peptide therapies, enhancing the industry's ability to address unmet medical needs while maintaining a competitive edge.
Restraints/Challenges
- High Production Costs
The production of peptide APIs can be expensive due to complex synthesis processes, the need for high-purity raw materials, and advanced purification techniques. These high production costs can limit the accessibility of peptide-based therapies, making it challenging for manufacturers to maintain competitive pricing in the market. Additionally, smaller biotech companies may struggle to invest in the necessary infrastructure and technologies, potentially hindering innovation and market entry for new peptide therapeutics.
For instance,
- Peptide API production demands significant costs and infrastructure, as it involves a complex, multi-step synthesis process with numerous chemical stages, followed by intensive purification efforts. These high production costs and intricate manufacturing requirements act as a restraint for the global Peptide API Market, limiting accessibility and increasing barriers for smaller manufacturers
High production costs present a significant restraint for the peptide API market, as they can hinder affordability, limit accessibility, and pose challenges for smaller companies attempting to innovate or compete. Addressing these cost barriers is essential to ensure broader adoption and sustained growth in the peptide therapeutics environment.
- Complexity of Peptide Design and Synthesis
The complexity of peptide design and synthesis poses a significant challenge in the global peptide API market. Peptides, often consisting of 20 or more amino acids, require precise sequence arrangement to achieve their desired therapeutic effects. This intricate design process involves balancing stability, solubility, and bioavailability, while minimizing immunogenicity and degradation by enzymes. Synthesizing these molecules further complicates production, as it demands advanced techniques like solid-phase or liquid-phase synthesis and stringent purification processes to achieve high purity and yield. Additionally, the synthesis of long-chain or cyclic peptides often leads to aggregation, misfolding, or low efficiency, increasing costs and time. Incorporating non-natural amino acids or complex modifications to enhance therapeutic properties adds another layer of difficulty. These challenges require expertise, cutting-edge technology, and significant investment, which can hinder the rapid development and commercialization of peptide-based drugs, especially for smaller or emerging players in the market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Peptide API Market Scope
The market is segmented on the basis of product type, therapeutic area, route of administration, source, and application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Hormonal Peptides
- Cyclic Peptides
- Linear Peptides
- Peptide Derivatives
Therapeutic Area
- Oncology
- Diabetes
- Cardiovascular
- Central Nervous System (CNS)
- Infectious Diseases, Others
Route of Administration
- Injectables
- Oral
- Others
Source
- Natural Sources
- Synthetic Sources
Application
- Research and Development
- Commercial Production
Peptide API Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, product type, therapeutic area, route of administration, source, and application as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its robust biotechnology sector, significant investment in research and development, advanced healthcare infrastructure, and a strong focus on innovation in peptide therapeutics.
Asia-Pacific is expected to be the fastest growing due to increasing healthcare expenditure, a rising prevalence of chronic diseases, growing biopharmaceutical industries, and expanding access to advanced therapeutic options in emerging economies.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Peptide API Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Peptide API Market Leaders Operating in the Market Are:
- Novo Nordisk A/S (Denmark)
- Bachem Holding AG (Switzerland)
- AstraZeneca (UK)
- Merck KGaA (Germany)
- Amgen Inc. (U.S.)
- H. Lundbeck A/S (Denmark)
- PeptiDream Inc. (Japan)
- PolyPeptide Group (France)
- Thermo Fisher Scientific Inc. (U.S.)
- AmbioPharm, Inc. (U.S.)
- CordenPharma International GmbH (Germany)
- Lonza Group Ltd. (Switzerland)
- CSBio Company, Inc. (U.S.)
- ChemPartner Co., Ltd. (China)
- Dr. Reddy's Laboratories Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Ferring Pharmaceuticals (Switzerland)
- Pfizer Inc. (U.S.)
Latest Developments in Peptide API Market
- In May 2024, Donaldson Company, Inc. and PolyPeptide Group AG have partnered to develop a large-scale solvent recovery system for peptide purification. Peptides, used as APIs, are expanding in therapeutic areas such as oncology, metabolic disorders, and neurology. This collaboration will enhance production efficiency, reducing costs and supporting the growing demand for peptide-based therapies
- In April 2024, CordenPharma and GENEPEP, in their 2023 partnership, are combining CordenPharma's cGMP manufacturing and regulatory expertise with GENEPEP's strengths in peptide drug discovery and development. This collaboration supports biotech companies in advancing peptides through early clinical phases. It will enhance the companies' capabilities in delivering high-quality peptide therapies while accelerating time-to-market for innovative treatments
- In March 2024, AstraZeneca has reached an agreement to acquire Amolyt Pharma, a clinical-stage biotech focused on rare endocrine diseases. This acquisition will strengthen the Alexion Rare Disease pipeline and expand its bone metabolism portfolio with eneboparatide (AZP-3601), a Phase III investigational peptide for hypoparathyroidism. It will enhance AstraZeneca’s position in rare disease treatments, particularly in bone metabolism, and support its growth in this specialized therapeutic area
- In January 2024, WuXi AppTec expanded its manufacturing capabilities with two new peptide plants in Changzhou and Taixing, China, increasing solid-phase peptide synthesis reactor volume to 32,000 L. The plants will feature digital systems and automated solvent delivery to optimize production and reduce cycle time. This expansion will enhance production efficiency, improve scalability, and help meet the growing demand for peptide-based therapeutics
- In November 2023, Novo Nordisk has announced plans to invest over 42 billion Danish kroner from 2023 onward to expand its manufacturing facilities in Kalundborg, Denmark, primarily focusing on increasing active pharmaceutical ingredient (API) capacity along with packaging capabilities. This investment is expected to enhance the company's global production capacity, enabling it to meet the rising demand for its products and support future growth
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.