Europe Stem Cell Therapy Market, By Product Type (Bone Marrow Derived Mesenchymal Cells, Placental or Umbilical Stem Cell, Adipose Tissue Derived Mesenchymal Stem Cells, and Others), Type (Allogenic Stem Cell Therapy and Autologous Stem Cell Therapy), Application (Musculoskeletal Disorders, Acute Graft-Versus-Host Disease (AGVHD), Wounds and Injuries, Cardiovascular Diseases, Surgeries, Gastrointestinal Diseases, and Others), End User (Hospitals and Surgical Centers, Therapeutic Companies, Services Companies, and Others), Distribution Channel (Direct Tender, Third Party Distributors) Industry Trends and Forecast to 2029
Market Analysis and Size
Chronic diseases-including cancer, musculoskeletal disorders and neurology disorders, chronic injuries, cardiovascular and gastrointestinal-can lead to hospitalization, long-term disability, reduced quality of life, and death.
The mesenchymal stem cells penetrate and integrate into multiple organs, repair cardiovascular, lung, and spinal cord injuries, and improve the state of autoimmune diseases, liver, and bone and cartilage diseases. Stems cells are a potent tool for the treatment of infections caused by inflammation, immune system failure, and, or tissue degeneration.
The drivers responsible for the growth of the Europe stem cell therapy market are the increased incidence of chronic diseases, rise in GMP-certification approvals for cell therapy production facilities, growing biotechnology sector and rise in clinical trials for stem-cell-based therapies. However, factors that are expected to restrain the market growth are the rise in the cost of stem cell-based research, and the risks faced while undergoing stem cell therapy, and the availability of alternatives.
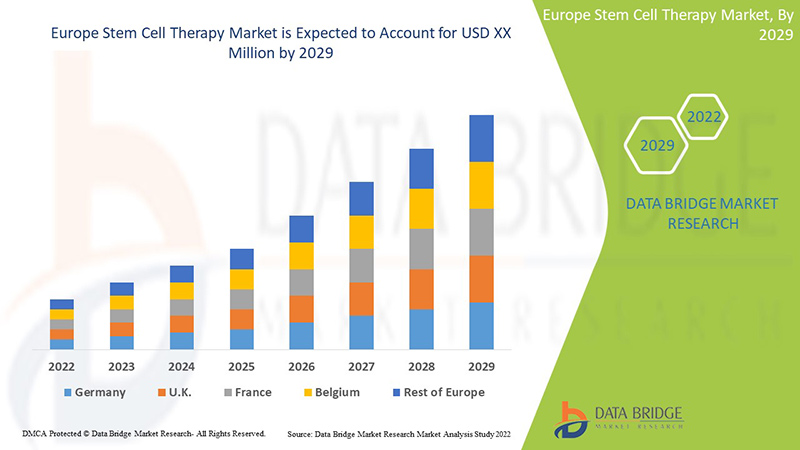
The Europe stem cell therapy is supportive and aims to reduce the severity of the symptoms. Data Bridge Market Research analyses that the Europe stem cell therapy market is expected to reach the value of USD 8.15 million and grow at a CAGR of 10.0% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Year |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Bone Marrow Derived Mesenchymal Cells, Placental or Umbilical Stem Cell, Adipose Tissue Derived Mesenchymal Stem Cells, and Others), Type (Allogenic Stem Cell Therapy and Autologous Stem Cell Therapy), Application (Musculoskeletal Disorders, Acute Graft-Versus-Host Disease (AGVHD), Wounds and Injuries, Cardiovascular Diseases, Surgeries, Gastrointestinal Diseases, and Others), End User (Hospitals and Surgical Centers, Therapeutic Companies, Services Companies, and Others), Distribution Channel (Direct Tender, Third Party Distributors) |
|
Countries Covered |
이탈리아, 벨기에, 체코, 독일, 영국, 스페인, 헝가리, 폴란드 |
|
시장 참여자 포함 |
Takeda Pharmaceutical Company Limited, Holostem Terapie Avanzate Srl, JCR Pharmaceuticals Co., Ltd, ANTEROGEN.CO., LTD, MEDIPOST, Orthofix Medical Inc., BioRestorative Therapies, Inc., STEMPEUTICS RESEARCH PVT LTD, Pluristem Inc. 등 |
시장 정의
줄기세포는 신체의 초기 물질로, 특수 기능을 가진 다른 모든 세포가 생성됩니다. 신체 또는 실험실에서 적절한 조건 하에 줄기세포는 분열하여 더 많은 딸세포라고 불리는 세포를 형성합니다. 딸세포는 혈액 세포, 뇌 세포, 심장 근육 세포 또는 뼈 세포와 같이 더 구체적인 기능을 가진 새로운 줄기세포 또는 특수 세포(분화)가 됩니다. 줄기세포에 대한 많은 관심은 연구 과학자들 사이에서 관심을 불러일으켰습니다. 줄기세포를 사용하여 질병이 어떻게 발병하고 발생하는지 이해하고, 세포를 대체할 건강한 세포를 생성하고, 신약의 안전성과 효능을 테스트하는 것이 줄기세포 치료제가 사용되는 과학적 이유입니다.
줄기세포 치료는 줄기세포 또는 파생물을 사용하여 기능 장애 또는 손상된 조직 복구 반응을 촉진합니다. 장기 이식 의 다음 장이며 공급이 제한적인 기증 장기 대신 세포를 사용합니다. 지방 조직 유래 중간엽 줄기 세포, 골수 유래 중간 엽 줄기세포, 태반 또는 탯줄 줄기세포와 같은 성인 줄기세포는 대부분 조직에서 적은 수로 발견됩니다. 배아 줄기세포는 3~5일 된 배아에서 유래합니다. 새로운 징후는 성인 줄기세포가 다양한 유형의 세포를 생성할 수 있음을 나타냅니다.
유럽 줄기세포 치료 시장 동향
운전자
- 만성질환의 유병률 및 발생률 증가
만성 질환은 전 세계적으로 흔한 건강 상태입니다. 유럽에서는 성인 3명 중 1명이 만성 질환을 앓고 있습니다. 만성 질환은 많은 시민의 건강과 삶의 질에 영향을 미쳤습니다. 암, 근골격계 질환 및 신경계 질환, 만성 손상, 심혈관 및 위장관 질환을 포함한 만성 질환은 입원, 장기 장애, 삶의 질 저하 및 사망으로 이어질 수 있습니다.
중간엽 줄기 세포는 여러 장기에 침투하여 통합되고, 심혈관, 폐 및 척수 손상을 치료하고, 자가면역 질환, 간, 뼈 및 연골 질환의 상태를 개선합니다. 줄기 세포는 염증, 면역 체계 부전 및 조직 변성으로 인한 질병을 치료하는 강력한 도구입니다.
예를 들어,
- 세계보건기구(WHO)에 따르면 2021년 전 세계적으로 근골격계 질환을 앓고 있는 사람이 약 17억 명이었습니다. 허리 통증은 근골격계 질환의 부담을 크게 증가시킵니다.
- 줄기세포 연구를 위한 연구개발 및 자금 지원의 증가
줄기세포 연구는 국립보건원(NIH) 예산으로 지원됩니다. 민간 부문도 줄기세포 연구에 자금을 지원하지만, 이러한 투자는 일반적으로 나중에, 테스트 및 개발 단계에서, 그 다음 초기 기초 연구에서 이루어집니다. 줄기세포 치료법이 매우 새로운 분야이기 때문에 편견 없는 정부 기관이 이를 감독해야 합니다. FDA는 신중하고 철저하지만, 그들은 끊임없이 자금 조달에 어려움을 겪고 있으며, 지불을 잠재적인 미래 수혜자와 일치시키는 장기 투자를 하고 있습니다.
예를 들어,
- 2021년 12월, 영국에서 MRC와 생명공학 및 BBSRC는 영국 줄기세포 은행에 자금을 지원하여 윤리적으로 승인되고 품질이 관리되며 인간 배아, 태아 및 성인 줄기세포 계통을 안전하게 보관하기 위한 연구 프로젝트를 수행했습니다.
- 세포치료 생산 시설에 대한 GMP 인증 승인 증가
GMP는 제품이 의도된 용도에 적합한 최첨단 품질 표준에 따라 일관되게 생산되고 관리되도록 보장합니다. 따라서 GMP 원칙은 소비자와 환자에게 일관된 품질과 높은 안전 수준의 제품을 제공하는 데 크게 기여합니다.
GMP 인증을 받으면 오류가 예방됩니다. 인증을 받으면 제조업체는 동일하고 반복 가능한 무균 환경에서 세포주를 생산할 수 있습니다. 따라서 GMP 인증은 유럽 줄기세포 치료 시장 성장을 촉진할 수 있습니다.
기회
- 의료비 지출 증가
게다가 정부와 민간 기관의 연구 개발 활동이 늘어나고 투자도 늘어나면서 시장 성장률에 새로운 기회가 생길 것입니다.
예를 들어,
- 2020년 유로스탯에 따르면 유럽연합의 의료비 지출은 1,309.6백만 달러에 달했습니다.
- 시장 참여자들의 전략적 이니셔티브
줄기세포 치료에 대한 수요는 만성 질환의 시기적절한 치료로 인해 유럽에서 수요를 증가시켰습니다. 이러한 유리한 요인은 약물에 대한 필요성을 강화하고, 시장 수요를 달성하기 위해 소규모 및 대규모 시장 참여자는 다양한 전략을 활용하고 있습니다.
주요 기업들은 또한 사업을 원활하게 운영하고, 위험을 피하고, 시장 판매의 장기적 성장을 높이기 위해 제품 출시, 인수, 승인, 확장, 파트너십 등의 구체적인 전략을 고안하려고 노력하고 있습니다.
인수, 컨퍼런스, 집중된 세그먼트 제품 출시를 포함한 시장 참여자들의 이러한 전략적 이니셔티브는 회사가 성장하고 회사의 제품 포트폴리오를 개선하는 데 도움이 되며 궁극적으로 더 많은 수익 창출로 이어집니다. 따라서 시장 참여자들의 이러한 전략적 이니셔티브는 시장 성장을 촉진하는 데 도움이 되는 기회를 제공합니다.
제약/도전
- 줄기세포 기반 치료 연구 비용 증가
줄기세포 치료는 여러 질환을 치료하기 위한 개발되고 새로운 치료 옵션입니다. 때때로, 치료 비용은 여러 질환에 대한 우려 사항입니다. 줄기세포 치료 치료 절차. 줄기세포 분야는 여전히 매우 전문화되어 있으며 주류 및 보험 회사에서 채택하지 않았습니다. 줄기세포 치료 기반 연구 치료 비용은 의료 보험에서 보장되지 않습니다. 이러한 비용은 환자에게 부담이 됩니다. 따라서 현재의 높은 비용은 감소 추세를 보일 것으로 예상됩니다.
예를 들어,
- 영국 자매 자선 단체인 Podari Zhizn의 Gift of Life가 2021년에 발표한 자료에 따르면 근골격계 질환에 대한 줄기세포 치료 비용은 4,861.44~5,401.60달러입니다.
- 줄기세포 치료를 받는 동안 직면하는 위험
줄기세포 치료사와 연구 과학자는 줄기세포 치료 모델을 개발하는 동안 특정 위험을 관찰합니다. 줄기세포 치료를 사용하는 동안 직면한 주요 과제는 최적의 세포 공급원입니다. 줄기세포 치료는 선진국에서 잘 확립되어 빠르게 성장하고 있지만, 특정 위험은 개발도상국에서 이 치료의 성장을 보호합니다. 기준은 줄기세포 치료의 사용을 제한합니다. 따라서 줄기세포 치료를 받는 환자에게는 특정 위험이 관찰됩니다.
예를 들어,
- 줄기세포 치료를 받는 동안 직면하는 위험에는 유전적 불안정성, 종양 형성, 부적절한 줄기세포 형성, 이식된 줄기세포의 면역 거부, 신경외과 수술 중 출혈, 수술 후 감염 등이 있습니다.
- 줄기세포 이식 후 직면하는 위험은 이식 후 줄기세포의 부적절한 분포 및 국소화입니다. 다능성 줄기세포는 기형종(종양)을 형성할 수 있습니다.
- 엄격한 규정
줄기세포 치료제에 대한 규제 지침은 이전 지침에 비해 엄격합니다. 제조업체는 승인 전에 특정 제품 변경을 해야 하며, 이로 인해 지연이 발생할 것으로 예상됩니다. 회사는 식품의약국(FDA)과 유럽연합(EU)에 따라 제품을 분류하기 전에 제품 사양과 인증을 신중하게 검토해야 합니다. 모든 국가에는 의약품의 개발, 허가, 등록, 제조, 마케팅 및 라벨링에 대한 규칙과 규정을 시행할 책임이 있는 규제 기관이 있습니다.
FDA의 생물학적 제제 평가 및 연구 센터(CBER)는 조혈 줄기 세포를 포함하여 이식을 목적으로 하는 인간 조직, 세포 및 조직 기반 제품을 규제합니다.
예를 들어,
- 영국에서는 의약품 및 의료제품 규제 기관(MHRA)이 줄기세포 치료를 규제하고 지침을 제공합니다. 줄기세포 치료가 의약품(MP)으로 간주되는 경우 품질 및 안전(Q 및 S)을 위한 테스트 규정이 적용됩니다.
유럽 줄기세포 치료 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 유럽 줄기세포 치료 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
환자 역학 분석
유럽 줄기세포 치료 시장은 또한 환자 분석, 예후 및 치료법에 대한 자세한 시장 분석을 제공합니다. 유병률, 발생률, 사망률 및 준수율은 보고서에서 사용할 수 있는 일부 데이터 변수입니다. 시장 성장에 대한 역학의 직접 또는 간접 영향 분석을 분석하여 성장 기간 동안 시장을 예측하기 위한 보다 견고하고 코호트 다변량 통계 모델을 만듭니다.
COVID-19가 유럽 줄기세포 치료 시장 에 미치는 영향
팬데믹 동안 줄기세포 치료는 COVID-19 환자의 사망률과 이환율을 줄이는 데 놀라운 효과가 있었습니다. 이러한 결과를 승인하려면 더 많은 대규모 연구가 필요합니다. 최상의 임상 결과를 달성하기 위해 COVID-19 감염에서 줄기세포 치료에 대한 프로토콜을 정의해야 합니다. COVID-19 동안 임상 시험이 수행되었습니다.
최근 개발
- 2020년 9월, Takeda Pharmaceutical Company Limited.는 비활성 또는 경미한 활성 루멘 크론병(CD) 환자의 복잡한 항문 주위 누공 치료를 위해 Alofisel(darvadstrocel) 및 지방 유래 중간엽 줄기 세포를 제조 및 판매하기 위해 일본 보건, 노동 및 복지부로부터 제품 승인을 받았습니다. 승인은 시판 후 승인 및 제품 출시로 이어질 것입니다. 이를 통해 시장 성장이 증가할 것으로 예상됩니다.
유럽 줄기세포 치료 시장 범위
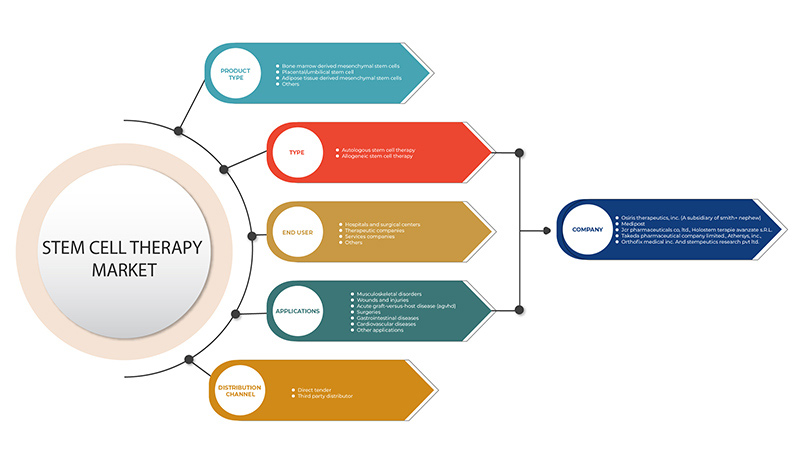
유럽 줄기세포 치료 시장은 제품 유형, 유형, 응용 분야, 최종 사용자 및 유통 채널을 기준으로 5개 세그먼트로 구분됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 분야를 식별하기 위한 전략적 결정을 내리는 데 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
제품 유형
- 골수유래 중간엽줄기세포
- 태반 또는 탯줄 줄기 세포
- 지방조직 유래 중간엽 줄기세포
- 기타
유럽 줄기세포 치료 시장은 제품 유형을 기준으로 골수 유래 중간엽 줄기세포, 태반 또는 태아줄기세포, 지방 조직 유래 중간엽 줄기세포 및 기타로 구분 됩니다 .
유형
- 동종 줄기 세포 치료
- 자가줄기세포 치료
유럽 줄기세포 치료 시장은 유형을 기준으로 동종줄기세포 치료와 자가줄기세포 치료로 구분됩니다.
애플리케이션
- 근골격계 질환
- 상처와 부상
- 급성 이식편대숙주병(AGVHD)
- 수술
- 위장관 질환
- 심혈관 질환
- 기타
유럽 줄기세포 치료 시장은 응용 분야를 기준으로 근골격계 질환, 상처 및 부상, 급성 이식편대숙주병(AGVHD), 수술, 위장관 질환 , 심혈관 질환 및 기타로 구분됩니다.
최종 사용자
- 병원 및 수술 센터
- 치료 회사
- 서비스 회사
- 기타
유럽 줄기세포 치료 시장은 최종 사용자를 기준으로 병원 및 수술 센터, 치료 회사, 서비스 회사 및 기타로 구분됩니다.
유통 채널
- 직접 입찰
- 제3자 유통업체
유럽 줄기세포 치료 시장은 유통 채널을 기준으로 직접 입찰과 제3자 유통업체로 구분됩니다.
유럽 줄기세포 치료 시장 지역 분석/통찰력
위에 언급된 대로 유럽 줄기세포 치료 시장을 분석하고, 지역, 제품 유형, 응용 분야, 최종 사용자 및 유통 채널별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
유럽 줄기세포 치료 시장 보고서에 포함된 국가로는 이탈리아, 벨기에, 체코, 독일, 영국, 스페인, 헝가리, 폴란드가 있습니다. 이탈리아는 인간 줄기세포 분야의 줄기세포 연구 및 개발에 대한 임상 시험 승인이 증가함에 따라 시장을 지배할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변경 사항을 제공합니다. 신규 판매, 교체 판매, 국가 인구 통계, 질병 역학 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 유럽 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 판매 채널의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 유럽 줄기세포 치료 시장 점유율 분석
The Europe stem cell therapy market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, the Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, application dominance. The above data points provided are only related to the companies' focus related to the Europe stem cell therapy market.
Some of the major players operating in the Europe stem cell therapy market are Takeda Pharmaceutical Company Limited, Holostem Terapie Avanzate S.r.l., JCR Pharmaceuticals Co., Ltd, ANTEROGEN.CO., LTD, MEDIPOST, Orthofix Medical Inc., BioRestorative Therapies, Inc., STEMPEUTICS RESEARCH PVT LTD, Pluristem Inc., among others.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or can drop down your inquiry. The key research methodology used by the DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Europe versus Regional, and Vendor Share Analysis. To know more about the research methodology, drop an inquiry to speak to our industry experts.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE STEM CELL THERAPY MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 EUROPE STEM CELL THERAPY MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR THE EUROPE STEM CELL THERAPY MARKET
7 EUROPE STEM CELL THERAPY MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 THE RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
8.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT AND AVAILABILITY OF FUNDING FOR STEM CELL RESEARCH
8.1.3 GROWING BIOTECHNOLOGY SECTOR
8.1.4 RISE IN GMP CERTIFICATION APPROVALS FOR CELL THERAPY PRODUCTION FACILITIES
8.1.5 RISE IN CLINICAL TRIALS FOR STEM-CELL-BASED THERAPIES
8.2 RESTRAINTS
8.2.1 THE RISE IN COST OF STEM-CELL-BASED THERAPY RESEARCH
8.2.2 THE RISKS FACED WHILE UNDERGOING STEM CELL THERAPY
8.2.3 ETHICAL CONCERNS RELATED TO STEM CELL THERAPY RESEARCH
8.2.4 AVAILABILITY OF ALTERNATIVES
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
8.3.2 RISE IN HEALTHCARE EXPENDITURE
8.3.3 THE EMERGENCE OF INDUCED PLURIPOTENT STEM CELLS (IPSCS)
8.4 CHALLENGES
8.4.1 THE LACK OF SKILLED PROFESSIONALS REQUIRED FOR STEM CELL THERAPY
8.4.2 STRINGENT REGULATIONS
9 EUROPE STEM CELL THERAPY MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 BONE MARROW DERIVED MESENCHYMAL STEM CELLS
9.3 PLACENTAL/UMBILICAL STEM CELL
9.4 ADIPOSE TISSUE DERIVED MESENCHYMAL STEM CELLS
9.5 OTHERS
10 EUROPE STEM CELL THERAPY MARKET, BY TYPE
10.1 OVERVIEW
10.2 ALLOGENEIC STEM CELL THERAPY
10.2.1 MUSCULOSKELETAL DISORDERS
10.2.2 WOUNDS AND INJURIES
10.2.3 ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD)
10.2.4 SURGERIES
10.2.5 GASTROINTESTINAL DISEASES
10.2.6 OTHER APPLICATION
10.3 AUTOLOGOUS STEM CELL THERAPY
10.3.1 CARDIOVASCULAR DISEASES
10.3.2 GASTROINTESTINAL DISEASES
10.3.3 OTHER APPLICATION
11 EUROPE STEM CELL THERAPY MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 MUSCULOSKELETAL DISORDERS
11.3 WOUNDS AND INJURIES
11.4 ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD)
11.5 SURGERIES
11.6 GASTROINTESTINAL DISEASES
11.7 CARDIOVASCULAR DISEASES
11.8 OTHER APPLICATION
12 EUROPE STEM CELL THERAPY MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL AND SURGICAL CENTERS
12.3 THERAPEUTIC COMPANIES
12.4 SERVICES COMPANIES
12.5 OTHERS
13 EUROPE STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTORS
14 EUROPE STEM CELL THERAPY MARKET, BY REGION
14.1 EUROPE
14.1.1 ITALY
14.1.2 BELGIUM
14.1.3 CZECH REPUBLIC
14.1.4 GERMANY
14.1.5 U.K.
14.1.6 SPAIN
14.1.7 HUNGARY
14.1.8 POLAND
15 EUROPE STEM CELL THERAPY MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 OSIRIS THERAPEUTICS, INC. (A SUBSIDIARY OF SMITH+NEPHEW) (2021)
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 JCR PHARMACEUTICALS CO., LTD ( (2021)
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 ORTHOFIX MEDICAL INC. (2021)
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.4 MEDIPOST (2021)
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.5 TAKEDA PHARMACEUTICAL COMPANY LIMITED (2021)
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CORESTEM, INC. (2021)
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 PHARMICELL CO., LTD. (2021)
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.8 ANTEROGEN.CO., LTD (2021)
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENTS
17.9 ATHERSYS, INC.(2021)
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
17.1 BRAINSTORM CELL LIMITED (2021)
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENTS
17.11 BIORESTORATIVE THERAPIES, INC. (2021)
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 HOLOSTEM TERAPIE AVANZATE S.R.L. (2021)
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENTS
17.13 INTERNATIONAL STEMCELL CORPORATION (2021)
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 MESOBLAST LTD (2021)
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENTS
17.15 PLURISTEM INC.(2021)
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 STEMPEUTICS RESEARCH PVT LTD
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENTS
17.17 U.S. STEM CELL, INC. (2021)
17.17.1 COMPANY SNAPSHOT
17.17.2 REVENUE ANALYSIS
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
표 목록
TABLE 1 EUROPE STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE BONE MARROW DERIVED MESENCHYMAL STEM CELLS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 EUROPE PLACENTAL/UMBILICAL STEM CELL IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE ADIPOSE TISSUE DERIVED MESENCHYMAL STEM CELLS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE OTHERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 EUROPE ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 EUROPE ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE MUSCULOSKELETAL DISORDERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE WOUNDS AND INJURIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD) IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE SURGERIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE GASTROINTESTINAL DISEASES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE CARDIOVASCULAR DISEASES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE OTHER APPLICATION IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 20 EUROPE HOSPITAL AND SURGICAL CENTERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE THERAPEUTIC COMPANIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE SERVICES COMPANIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE OTHERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 25 EUROPE DIRECT TENDER IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE THIRD PARTY DISTRIBUTORS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE STEM CELL THERAPY MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 28 EUROPE STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 29 EUROPE STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 30 EUROPE ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 33 EUROPE STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 34 EUROPE STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 35 ITALY STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 36 ITALY STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 37 ITALY ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 38 ITALY AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 39 ITALY STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 40 ITALY STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 ITALY STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 42 BELGIUM STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 43 BELGIUM STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 BELGIUM ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 45 BELGIUM AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 46 BELGIUM STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 47 BELGIUM STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 BELGIUM STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 49 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 50 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 51 CZECH REPUBLIC ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 52 CZECH REPUBLIC AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 53 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 54 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 CZECH REPUBLIC STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 GERMANY STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 57 GERMANY STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 58 GERMANY ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 59 GERMANY AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 60 GERMANY STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 61 GERMANY STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 62 GERMANY STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 63 U.K. STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 64 U.K. STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 65 U.K. ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 66 U.K. AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 U.K. STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 68 U.K. STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 69 U.K. STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 70 SPAIN STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 SPAIN STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 72 SPAIN ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 73 SPAIN AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 SPAIN STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 75 SPAIN STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 76 SPAIN STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 77 HUNGARY STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 HUNGARY STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 HUNGARY ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 80 HUNGARY AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 81 HUNGARY STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 82 HUNGARY STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 83 HUNGARY STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 84 POLAND STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 85 POLAND STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 86 POLAND ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 87 POLAND AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 88 POLAND STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 89 POLAND STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 90 POLAND STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 EUROPE STEM CELL THERAPY MARKET : SEGMENTATION
FIGURE 2 EUROPE STEM CELL THERAPY MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE STEM CELL THERAPY MARKET: DROC ANALYSIS
FIGURE 4 EUROPE STEM CELL THERAPY MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE STEM CELL THERAPY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE STEM CELL THERAPY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE STEM CELL THERAPY MARKET: DBMR POSITION GRID
FIGURE 8 EUROPE STEM CELL THERAPY MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE STEM CELL THERAPY MARKET: END USER COVERAGE GRID
FIGURE 10 EUROPE STEM CELL THERAPY MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE EUROPE STEM CELL THERAPY MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN CLINICAL TRIALS, GMP CERTIFICATION AND PRODUCT APPPROVALS IS EXPECTED TO DRIVE THE EUROPE STEM CELL THERAPY MARKET FROM 2022 TO 2029
FIGURE 13 PRODUCT TYPE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE EUROPE STEM CELL THERAPY MARKET FROM 2022 & 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE STEM CELL THERAPY MARKET
FIGURE 15 NUMBER AND AGES OF PEOPLE 65 OR OLDER WITH ALZHEIMER'S DEMENTIA IN 2022
FIGURE 16 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 17 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, 2021
FIGURE 18 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 19 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 20 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 21 EUROPE STEM CELL THERAPY MARKET: BY TYPE, 2021
FIGURE 22 EUROPE STEM CELL THERAPY MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 23 EUROPE STEM CELL THERAPY MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 24 EUROPE STEM CELL THERAPY MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, 2021
FIGURE 26 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 27 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 28 EUROPE STEM CELL THERAPY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 29 EUROPE STEM CELL THERAPY MARKET: BY END USER, 2021
FIGURE 30 EUROPE STEM CELL THERAPY MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 31 EUROPE STEM CELL THERAPY MARKET: BY END USER, CAGR (2022-2029)
FIGURE 32 EUROPE STEM CELL THERAPY MARKET: BY END USER, LIFELINE CURVE
FIGURE 33 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 34 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 35 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 36 EUROPE STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 37 EUROPE STEM CELL THERAPY MARKET: SNAPSHOT (2021)
FIGURE 38 EUROPE STEM CELL THERAPY MARKET: BY COUNTRY (2021)
FIGURE 39 EUROPE STEM CELL THERAPY MARKET: BY COUNTRY (2022 & 2029)
FIGURE 40 EUROPE STEM CELL THERAPY MARKET: BY COUNTRY (2021 & 2029)
FIGURE 41 EUROPE STEM CELL THERAPY MARKET: BY PRODUCT TYPE (2022 & 2029)
FIGURE 42 EUROPE STEM CELL THERAPY MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













